Abstract
Purpose of Review
This review highlights our current knowledge of oxygen tensions in the bone marrow, and how low oxygen tensions (hypoxia) regulate tumor metastasis to and colonization of the bone marrow.
Recent Findings
The bone marrow is a relatively hypoxic microenvironment, but oxygen tensions fluctuate throughout the marrow cavity and across the endosteal and periosteal surfaces. Recent advances in imaging have made it possible to better characterize these fluctuations in bone oxygenation, but technical challenges remain. We have compiled evidence from multiple groups that suggests that hypoxia or hypoxia inducible factor (HIF) signaling may induce spontaneous metastasis to the bone and promote tumor colonization of bone, particularly in the case of breast cancer dissemination to the bone marrow.
Summary
We are beginning to understand oxygenation patterns within the bone compartment and the role for hypoxia and HIF signaling in tumor cell dissemination to the bone marrow, but further studies are warranted.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer res. 1998;58(7):1408–16.
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2002;8(6):1831–7.
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573–81. doi:10.1002/cncr.11246.
Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, et al. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. International Journal of Cancer Journal International du Cancer. 2005;116(5):734–9. doi:10.1002/ijc.20984.
Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, et al. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2006;12(15):4562–8. doi:10.1158/1078-0432.CCR-05-2690.
Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe T, Kai K, Iyama K, et al. Hypoxia-inducible factor 1alpha is closely linked to an aggressive phenotype in breast cancer. Breast Cancer res Treat. 2008;110(3):465–75. doi:10.1007/s10549-007-9742-1.
Jin Y, Wang H, Ma X, Liang X, Liu X, Wang Y. Clinicopathological characteristics of gynecological cancer associated with hypoxia-inducible factor 1alpha expression: a meta-analysis including 6,612 subjects. PLoS One. 2015;10(5):e0127229. doi:10.1371/journal.pone.0127229.
Matsuo Y, Ding Q, Desaki R, Maemura K, Mataki Y, Shinchi H, et al. Hypoxia inducible factor-1 alpha plays a pivotal role in hepatic metastasis of pancreatic cancer: an immunohistochemical study. J Hepatobiliary Pancreat Sci. 2014;21(2):105–12. doi:10.1002/jhbp.6.
Ping W, Sun W, Zu Y, Chen W, Fu X. Clinicopathological and prognostic significance of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma: a meta-analysis. Tumour Biol. 2014;35(5):4401–9. doi:10.1007/s13277-013-1579-0.
Luan Y, Gao C, Miao Y, Li Y, Wang Z, Qiu X. Clinicopathological and prognostic significance of HIF-1alpha and HIF-2alpha expression in small cell lung cancer. Pathol res Pract. 2013;209(3):184–9. doi:10.1016/j.prp.2012.10.017.
Wang HX, Qin C, Han FY, Wang XH, Li N. HIF-2alpha as a prognostic marker for breast cancer progression and patient survival. Genet Mol res. 2014;13(2):2817–26. doi:10.4238/2014.January.22.6.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat rev Cancer. 2011;11(6):411–25. doi:10.1038/nrc3055.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54.
Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40. doi:10.1126/science.1066373.
Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S a. 2002;99(21):13459–64. doi:10.1073/pnas.192342099.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi:10.1038/20459.
Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO j. 2000;19(16):4298–309. doi:10.1093/emboj/19.16.4298.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi:10.1126/science.1059796.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi:10.1126/science.1059817.
Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, et al. Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem. 2003;278(13):11032–40. doi:10.1074/jbc.M208681200.
Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117(23):e207–17. doi:10.1182/blood-2010-10-314427.
Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S a. 1993;90(9):4304–8.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S a. 1995;92(12):5510–4.
Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175–80. doi:10.1126/science.aaf4405.
Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–56.
Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. Journal of Bone and Mineral Research : the Official Journal of the American Society for Bone and Mineral Research. 2012;27(3):499–505. doi:10.1002/jbmr.1547.
Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer res. 2015;75(11):2151–8. doi:10.1158/0008-5472.CAN-14-2493.
• Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat rev Cancer. 2016;16(6):373–86. doi:10.1038/nrc.2016.44. A nice recent review of tumor metastasis and dormancy in the bone marrow.
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–61. doi:10.1016/j.stem.2010.07.007.
•• Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. doi:10.1038/nature13034. Showed quantitatively for the first time differences in oxygen tensions within different regions of the calvaria.
Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys J. 2001;81(2):675–84.
Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99(1):394.
Rankin EB, Giaccia AJ, Schipani E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Current Osteoporosis Reports. 2011;9(2):46–52. doi:10.1007/s11914-011-0047-2.
Branemark P-I. Experimental investigation of microcirculation in bone marrow. Angiology. 1961;12(7):293–305.
• Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8. doi:10.1038/nature13145. Identified different vessel types in the bone, which link angiogenesis and osteogenesis, and show that these blood vessels decline with age.
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S a. 2007;104(13):5431–6. doi:10.1073/pnas.0701152104.
Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. doi:10.1002/jcp.21908.
Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–80. doi:10.1038/nature13146.
Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149(1):63–74. doi:10.1016/j.cell.2012.01.051.
Marenzana M, Arnett TR. The key role of the blood supply to bone. Bone res. 2013;1(3):203–15. doi:10.4248/BR201303001.
Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–43. doi:10.1038/ncb2730.
Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–85. doi:10.1182/blood-2009-07-233437.
Lassailly F, Foster K, Lopez-Onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122(10):1730–40. doi:10.1182/blood-2012-11-467498.
Zahm AM, Bucaro MA, Ayyaswamy PS, Srinivas V, Shapiro IM, Adams CS, et al. Numerical modeling of oxygen distributions in cortical and cancellous bone: oxygen availability governs osteonal and trabecular dimensions. Am J Physiol Cell Physiol. 2010;299(5):C922–9. doi:10.1152/ajpcell.00465.2009.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi:10.1038/nature12984.
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–6. doi:10.1038/nature07434.
Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, et al. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell. 2013;13(2):175–89. doi:10.1016/j.stem.2013.06.015.
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi:10.1038/ncb1691.
Finger EC, Castellini L, Rankin EB, Vilalta M, Krieg AJ, Jiang D, et al. Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc Natl Acad Sci U S A. 2015;112(14):4441–6. doi:10.1073/pnas.1418164112.
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–6. doi:10.1038/nature04695.
Chaturvedi P, Gilkes DM, Wong CC, Kshitiz LW, Zhang H, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123(1):189–205. doi:10.1172/JCI64993.
Woelfle U, Cloos J, Sauter G, Riethdorf L, Janicke F, van Diest P, et al. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer res. 2003;63(18):5679–84.
Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer res. 2007;67(2):563–72. doi:10.1158/0008-5472.CAN-06-2701.
Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi:10.1016/j.ccr.2007.12.003.
Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer res. 2007;67(9):4157–63. doi:10.1158/0008-5472.CAN-06-2355.
Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4(9):e6896. doi:10.1371/journal.pone.0006896.
Muz B, de la Puente P, Azab F, Luderer M, Azab AK. Hypoxia promotes stem cell-like phenotype in multiple myeloma cells. Blood Cancer J. 2014;4:e262. doi:10.1038/bcj.2014.82.
Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–94. doi:10.1182/blood-2011-09-380410.
Imai T, Muz B, Yeh CH, Yao J, Zhang R, Azab AK, et al. Direct measurement of hypoxia in a xenograft multiple myeloma model by optical-resolution photoacoustic microscopy. Cancer Biol Ther. 2017;18(2):101–5. doi:10.1080/15384047.2016.1276137.
Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi:10.1016/j.ccr.2008.11.012.
Graham CH, Forsdike J, Fitzgerald CJ, Macdonald-Goodfellow S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. International Journal of Cancer Journal International du Cancer. 1999;80(4):617–23.
Buchler P, Reber HA, Tomlinson JS, Hankinson O, Kallifatidis G, Friess H, et al. Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer. Neoplasia. 2009;11(2):196–206.
Allgayer H, Heiss MM, Riesenberg R, Grutzner KU, Tarabichi A, Babic R, et al. Urokinase plasminogen activator receptor (uPA-R): one potential characteristic of metastatic phenotypes in minimal residual tumor disease. Cancer res. 1997;57(7):1394–9.
Heiss MM, Allgayer H, Gruetzner KU, Funke I, Babic R, Jauch KW, et al. Individual development and uPA-receptor expression of disseminated tumour cells in bone marrow: a reference to early systemic disease in solid cancer. Nat med. 1995;1(10):1035–9.
Allgayer H, Heiss MM, Riesenberg R, Babic R, Jauch KW, Schildberg FW. Immunocytochemical phenotyping of disseminated tumor cells in bone marrow by uPA receptor and CK18: investigation of sensitivity and specificity of an immunogold/alkaline phosphatase double staining protocol. J Histochem Cytochem. 1997;45(2):203–12.
Allgayer H, Aguirre-Ghiso JA. The urokinase receptor (u-PAR)—a link between tumor cell dormancy and minimal residual disease in bone marrow? APMIS. 2008;116(7–8):602–14. doi:10.1111/j.1600-0463.2008.00997.x.
Yu W, Kim J, Ossowski L. Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J Cell Biol. 1997;137(3):767–77.
• Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y, et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016;18(10):1078–89. doi:10.1038/ncb3408. Identified a hypoxia-dependent role for LIFR in breast cancer dormancy in the bone marrow.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98(3):295–303.
Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE Jr. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18(5):2553–8.
Watson CJ, Miller WR. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. Br J Cancer. 1995;71(4):840–4.
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20(20):2499–513. doi:10.1038/sj.onc.1204349.
Wake MS, Watson CJ. STAT3 the oncogene—still eluding therapy? FEBS j. 2015;282(14):2600–11. doi:10.1111/febs.13285.
Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor cell dormancy. Recent Results Cancer res. 2012;195:25–39. doi:10.1007/978-3-642-28160-0_3.
• Fluegen G, Avivar-Valderas A, Wang Y, Padgen MR, Williams JK, Nobre AR, et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. 2017;19(2):120–32. doi:10.1038/ncb3465. Found that tumor hypoxia creates a distinct population of DTCs that are resistant to chemotherapy and may give rise to distant metastases.
Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, et al. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun. 2015;6:6170. doi:10.1038/ncomms7170.
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–17. doi:10.1038/ncb2767.
Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, et al. The BMP inhibitor coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150(4):764–79. doi:10.1016/j.cell.2012.06.035.
Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15(11):1351–61. doi:10.1038/ncb2861.
• Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11(1):40–61. doi:10.1002/1878-0261.12022. A nice recent review of tumor dormancy across multiple tumor types and tissues.
Acknowledgements
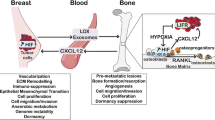
The authors wish to acknowledge Ms. Lauren Holtslander for the graphic design in Fig. 1. This manuscript was supported by R00CA194198 (RWJ) and CA67166, CA198291, and CA197713 (AJG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Miranda Sowder, Rachelle Johnson, and Amato Giaccia declare no conflicts of interests.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Cancer-induced Musculoskeletal Diseases
Rights and permissions
About this article
Cite this article
Johnson, R.W., Sowder, M.E. & Giaccia, A.J. Hypoxia and Bone Metastatic Disease. Curr Osteoporos Rep 15, 231–238 (2017). https://doi.org/10.1007/s11914-017-0378-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0378-8