Abstract
Purpose of Review
The term renal osteodystrophy has been used to describe a wide variety of bone problems facing patients with chronic kidney disease (CKD). Here, we review the history of the use of this term.
Recent Findings
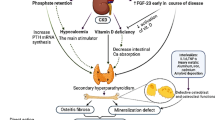
Bone disease resulting from CKD was first noticed in 1890. The term “renal osteodystrophy” was used to define the bone disease in 1942. Since then, important discoveries have increased our knowledge of the complexities of bone physiology in these patients. At the same time, secular changes in the disease have occurred. The terms used to describe the bone histological findings have changed as well, reflecting new understanding of the physiological processes. However, since different investigators used the terms in different ways, the need to standardize the nomenclature has become increasingly important.
Summary
Ongoing international collaboration about nosography will allow more optimal communication among scientists and clinicians as we continue to make new discoveries.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Christison R. On granular degeneration of the kidnies, and its connexion with dropsy, inflammations, and other diseases. Edinburgh: Adam and Charles Black; 1839. p. 1–288.
Lucas R. Form of late rickets associated with albuminuria, rickets of adolescents. Lancet. 1883;1:993–4.
Malluche H, Monier-Faugere MC. Renal osteodystrophy: what’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol. 2006;65:235–42.
Fletcher H. Case of infantilism with polyuria and chronic renal disease. Proc Roy Soc Med. 1911;4:95.
Brockman E. Some observations on the bone changes in renal rickets. Br J Surgery. 1926;14:634–45.
Lightwood R. Renal Rickets. Proc Roy Soc Med. 1932;25:418–20.
Abright F, Drake T, Sulkowitch H. Renal osteitis fibrosa cystica: report of a case. Bull Johns Hopkins Hosp. 1937;60:377–99.
Liu SH, Chu HI. Treatment of renal osteodystrophy with dihydrotachysterol (A.T.10) and iron. Science. 1942;95(2467):388–9.
Liu S, Chu H. Studies of calcium and phosphorus metabolism with special reference to pathogenesis and effects of dihydrotachysterol and iron. Medicine (Baltimore). 1943;22:103–61.
Stanbury S. Azotaemic renal osteodystrophy. Br Med Bull. 1957;13:57–60.
Pendras JP, Erickson RV. Hemodialysis: a successful therapy for chronic uremia. Ann Intern Med. 1966;64(2):293–311.
Slatopolsky E, Gradowska L, Kashemsant C, Keltner R, Manley C, Bricker N. The control of phosphate excretion in uremia. J Clin Invest. 1966;45:672–7.
Slatopolsky E, Caglar S, Gradowska L, Canterbury J, Reiss E, Bricker NS. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using “proportional reduction” of dietary phosphorus intake. Kidney Int. 1972;2(3):147–51.
Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973;4(2):141–5.
Frost HM. Tetracycline-base histological analysis of bone remodeling. Calc Tiss Res. 1969;3:211–37.
Hitt O, Jaworski ZF, Shimizu AG, Frost HM. Tissue-level bone formation rates in chronic renal failure, measured by means of tetracycline bone labeling. Can J Physiol Pharmacol. 1970;48(12):824–8.
Sherrard DJ, Baylink DJ, Wergedal JE, Maloney NA. Quantitative histological studies on the pathogenesis of uremic bone disease. J Clin Endocrinol Metab. 1974;39(1):119–35.
Malluche HH, Ritz E, Lange HP, Kutschera J, Hodgson M, Seiffert U, et al. Bone histology in incipient and advanced renal failure. Kidney Int. 1976;9(4):355–62.
Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-Dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci U S A. 1971;68:803–4.
Lawson DEM, Fraser DR, Kodicek E, Morris HR, Williams DH. Identification of 1,25-Dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971;230:228–30.
Brickman AS, Sherrard DJ, Jowsey J, Singer FR, Baylink DJ, Maloney N, et al. 1,25-dihydroxycholecalciferol. Effect on skeletal lesions and plasma parathyroid hormone levels in uremic osteodystrophy. Arch Intern Med. 1974;134(5):883–8.
Ellis HA, Pierides AM, Feest TG, Ward MK, Kerr DN. Histopathology of renal osteodystrophy with particular reference to the effects of 1alpha-hydroxyvitamin D3 in patients treated by long-term haemodialysis. Clin Endocrinol. 1977;7:Suppl:31s–8s.
Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294(4):184–8.
Hodsman AB, Sherrard DJ, Alfrey AC, Ott S, Brickman AS, Miller NL, et al. Bone aluminum and histomorphometric features of renal osteodystrophy. J Clin Endocrinol Metab. 1982;54(3):539–46.
Ott SM, Maloney NA, Coburn JW, Alfrey AC, Sherrard DJ. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N Engl J Med. 1982;307:709–13.
• London GM. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15(7):1943–51. This study demonstrated that patients with arterial calcifications were more likely to have low bone formation rates, which led to recognition that vascular disease was related to bone disease in CKD patients
Barreto DV, Barreto Fde C, Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52(6):1139–50.
Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85(1):142–50.
Malluche HH, Blomquist G, Monier-Faugere MC, Cantor TL, Davenport DL. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol. 2015;26(10):2534–44.
Gonzalez EA, Lund RJ, Martin KJ, McCartney JE, Tondravi MM, Sampath TK, et al. Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int. 2002;61(4):1322–31.
Lotinun S, Sibonga JD, Turner RT. Evidence that the cells responsible for marrow fibrosis in a rat model for hyperparathyroidism are preosteoblasts. Endocrinology. 2005;146(9):4074–81.
Calvi L. Activated parathyroid hormone/PTHRP in osteoblastic cells differentially affects cortical and trabecular bone. JCI. 2001;107(3):277–86.
Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89(2):289–302.
Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, et al. The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int. 1993;43:436–42.
• Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26(6):1368–76. This study showed the shifting patterns of renal osteodystrophy and highlighted the use of newer classification systems
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Mineral Res. 1987;2:595–610.
• Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. The ASBMR nomenclature standardizations have been internationally accepted and have helped scientists communicate their results
National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201.
• Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945–53. This was a key paper discussing the relationships between mineral metabolism and bone disease and vascular disease in patients with CKD. Recognizing these relationships, physicians must examine whether drugs to treat bone disease could affect vascular disease and whether drugs aimed at vascular disease could also have effects on the skeleton
Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009;76(113):S1–130.
Ott SM. Histomorphometric measurements of bone turnover, mineralization, and volume. Clin J Am Soc Nephrol. 2008;3:S151–6.
Ott SM. Bone strength: more than just bone density. Kidney Int. 2016;89(1):16–9.
Liu ES, Martins JS, Raimann A, Chae BT, Brooks DJ, Jorgetti V, et al. 1,25-Dihydroxyvitamin D alone improves skeletal growth, microarchitecture, and strength in a murine model of XLH, despite enhanced FGF23 expression. J Bone Miner Res. 2016;31(5):929–39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Susan Ott declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Kidney and Bone
Rights and permissions
About this article
Cite this article
Ott, S.M. Renal Osteodystrophy—Time for Common Nomenclature. Curr Osteoporos Rep 15, 187–193 (2017). https://doi.org/10.1007/s11914-017-0367-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0367-y