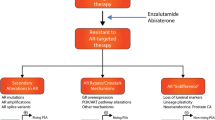
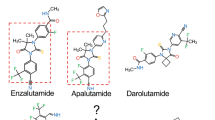
Abstract
Purpose of Review
Treatment-emergent neuroendocrine prostate cancer (NEPC) is aggressive and lethal. As androgen receptor signaling inhibitors (ARSIs) are increasingly used in earlier disease settings, treatment-emergent NEPC becomes more prevalent, and effective therapies are urgently needed. The purpose of this review was to summarize recent progress on emerging therapeutic targets of NEPC.
Recent Findings
A multitude of therapeutic targets have emerged in NEPC over recent years. These targets may represent drivers of treatment-emergent lineage plasticity or simply be overexpressed on the surface of NEPC cells. Multiple modalities have been employed to drug these targets, with promising preclinical and clinical results.
Summary
Treatment-emergent NEPC represents a distinct and clinically significant subset of castration-resistant prostate cancer (CRPC). Emerging therapeutic approaches have demonstrated encouraging efficacy and safety profiles, offering the potential to improve patient outcomes.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Yamada Y, Beltran H. Clinical and biological features of neuroendocrine prostate cancer. Curr Oncol Rep. 2021;23:15.
Haffner MC, Morris MJ, Ding C-KC, Sayar E, Mehra R, Robinson B et al. Framework for the pathology workup of metastatic castration-resistant prostate cancer biopsies. Clin Cancer Res [Internet]. 2024; Available from: https://doi.org/10.1158/1078-0432.CCR-24-2061
Eule CJ, Hu J, Al-Saad S, Collier K, Boland P, Lewis AR, et al. Outcomes of second-line therapies in patients with metastatic de Novo and treatment-emergent neuroendocrine prostate cancer: a multi-institutional study. Clin Genitourin Cancer. 2023;21:483–90.
Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate Cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36:2492–503.
Quintanal-Villalonga Á, Chan JM, Yu HA, Pe’er D, Sawyers CL, Sen T, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17:360–71.
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
Rubin MA, Bristow RG, Thienger PD, Dive C, Imielinski M. Impact of lineage plasticity to and from a neuroendocrine phenotype on progression and response in prostate and lung cancers. Mol Cell. 2020;80:562–77.
Lundberg A, Zhang M, Aggarwal R, Li H, Zhang L, Foye A, et al. The genomic and Epigenomic Landscape of double-negative metastatic prostate Cancer. Cancer Res. 2023;83:2763–74.
Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83.
Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen C-C, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8.
Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a mechanism of Treatment Resistance in a mouse model of castration-resistant prostate Cancer. Cancer Discov. 2017;7:736–49.
Park JW, Lee JK, Sheu KM, Wang L, Balanis NG, Nguyen K, et al. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science. 2018;362:91–5.
Romero R, Chu T, González Robles TJ, Smith P, Xie Y, Kaur H et al. The neuroendocrine transition in prostate cancer is dynamic and dependent on ASCL1. Nat Cancer. 2024;1–19.
Aparicio AM, Shen L, Tapia ELN, Lu J-F, Chen H-C, Zhang J, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–30.
Corn PG, Heath EI, Zurita A, Ramesh N, Xiao L, Sei E, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1–2 trial. Lancet Oncol. 2019;20:1432–43.
Cyrta J, Augspach A, De Filippo MR, Prandi D, Thienger P, Benelli M, et al. Role of specialized composition of SWI/SNF complexes in prostate cancer lineage plasticity. Nat Commun. 2020;11:5549.
Xiao L, Parolia A, Qiao Y, Bawa P, Eyunni S, Mannan R, et al. Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature. 2022;601:434–9.
Li JJ, Vasciaveo A, Karagiannis D, Sun Z, Chen X, Socciarelli F et al. NSD2 maintains lineage plasticity and castration-resistance in neuroendocrine prostate cancer. bioRxivorg. 2023;2023.07.18.549585.
Parolia A, Eyunni S, Verma BK, Young E, Liu Y, Liu L et al. NSD2 is a requisite subunit of the AR/FOXA1 neo-enhanceosome in promoting prostate tumorigenesis. Nat Genet. 2024;1–12.
Adams EJ, Karthaus WR, Hoover E, Liu D, Gruet A, Zhang Z, et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature. 2019;571:408–12.
Baca SC, Takeda DY, Seo J-H, Hwang J, Ku SY, Arafeh R, et al. Reprogramming of the FOXA1 cistrome in treatment-emergent neuroendocrine prostate cancer. Nat Commun. 2021;12:1979.
Han M, Li F, Zhang Y, Dai P, He J, Li Y, et al. FOXA2 drives lineage plasticity and KIT pathway activation in neuroendocrine prostate cancer. Cancer Cell. 2022;40:1306–e13238.
Nouruzi S, Namekawa T, Tabrizian N, Kobelev M, Sivak O, Scurll JM et al. ASCL1 regulates and cooperates with FOXA2 to drive terminal neuroendocrine phenotype in prostate cancer. JCI Insight [Internet]. 2024 [cited 2024 Oct 31]; Available from: https://pubmed.ncbi.nlm.nih.gov/39470735/
Nouruzi S, Ganguli D, Tabrizian N, Kobelev M, Sivak O, Namekawa T, et al. ASCL1 activates neuronal stem cell-like lineage programming through remodeling of the chromatin landscape in prostate cancer. Nat Commun. 2022;13:2282.
Chen C-C, Tran W, Song K, Sugimoto T, Obusan MB, Wang L, et al. Temporal evolution reveals bifurcated lineages in aggressive neuroendocrine small cell prostate cancer trans-differentiation. Cancer Cell. 2023;41:2066–82.e9.
Rodarte KE, Nir Heyman S, Guo L, Flores L, Savage TK, Villarreal J et al. Neuroendocrine differentiation in prostate cancer requires ASCL1. Cancer Res [Internet]. 2024 [cited 2024 Oct 11]; Available from: https://pubmed.ncbi.nlm.nih.gov/39264686/
Varuzhanyan G, Chen C-C, Freeland J, He T, Tran W, Song K et al. PGC-1α drives small cell neuroendocrine cancer progression towards an ASCL1-expressing subtype with increased mitochondrial capacity. bioRxivorg [Internet]. 2024 [cited 2024 Oct 11]; Available from: https://pubmed.ncbi.nlm.nih.gov/38645232/
Lee JK, Phillips JW, Smith BA, Park JW, Stoyanova T, McCaffrey EF, et al. N-Myc drives neuroendocrine prostate Cancer initiated from human prostate epithelial cells. Cancer Cell. 2016;29:536–47.
Xu P, Yang JC, Chen B, Ning S, Zhang X, Wang L, et al. Proteostasis perturbation of N-Myc leveraging HSP70 mediated protein turnover improves treatment of neuroendocrine prostate cancer. Nat Commun. 2024;15:6626.
Qian C, Yang Q, Rotinen M, Huang R, Kim H, Gallent B, et al. ONECUT2 acts as a lineage plasticity driver in adenocarcinoma as well as neuroendocrine variants of prostate cancer. Nucleic Acids Res. 2024;52:7740–60.
Xu Y, Vakoc CR. Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harb Perspect Med. 2017;7:a026674.
Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82.
Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, et al. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of prostate cancer. Mol Cancer Res. 2016;14:324–31.
Janouskova H, El Tekle G, Bellini E, Udeshi ND, Rinaldi A, Ulbricht A, et al. Opposing effects of cancer-type-specific SPOP mutants on BET protein degradation and sensitivity to BET inhibitors. Nat Med. 2017;23:1046–54.
Zhang P, Wang D, Zhao Y, Ren S, Gao K, Ye Z, et al. Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation. Nat Med. 2017;23:1055–62.
Dai X, Gan W, Li X, Wang S, Zhang W, Huang L, et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat Med. 2017;23:1063–71.
Aggarwal RR, Schweizer MT, Nanus DM, Pantuck AJ, Heath EI, Campeau E, et al. A phase Ib/IIa study of the pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2020;26:5338–47.
Aggarwal R, Starodub AN, Koh BD, Xing G, Armstrong AJ, Carducci MA. Phase 1b study of the BET inhibitor GS-5829 as monotherapy and combined with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res [Internet]. 2022; Available from: https://doi.org/10.1158/1078-0432.CCR-22-0175
Study Details. NCT04471974 [Internet]. [cited 2024 Nov 10]. Available from: https://clinicaltrials.gov/study/NCT04471974
Kim D-H, Sun D, Storck WK, Welker Leng K, Jenkins C, Coleman DJ, et al. BET bromodomain inhibition blocks an AR-Repressed, E2F1-Activated treatment-emergent neuroendocrine prostate cancer lineage plasticity program. Clin Cancer Res. 2021;27:4923–36.
Choo N, Keerthikumar S, Ramm S, Ashikari D, Teng L, Niranjan B, et al. Co-targeting BET, CBP, and p300 inhibits neuroendocrine signalling in androgen receptor-null prostate cancer. J Pathol. 2024;263:242–56.
Faivre EJ, McDaniel KF, Albert DH, Mantena SR, Plotnik JP, Wilcox D, et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature. 2020;578:306–10.
Shen L, Wang B, Wang S-P, Ji S-K, Fu M-J, Wang S-W, et al. Combination therapy and dual-target inhibitors based on LSD1: New emerging tools in cancer therapy. J Med Chem. 2024;67:922–51.
Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AHFM, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–9.
Gao S, Chen S, Han D, Wang Z, Li M, Han W, et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat Genet. 2020;52:1011–7.
Nyquist MD, Corella A, Coleman I, De Sarkar N, Kaipainen A, Ha G, et al. Combined TP53 and RB1 loss promotes prostate Cancer Resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 2020;31:107669.
Han W, Liu M, Han D, Li M, Toure AA, Wang Z, et al. RB1 loss in castration-resistant prostate cancer confers vulnerability to LSD1 inhibition. Oncogene. 2022;41:852–64.
Li M, Liu M, Han W, Wang Z, Han D, Patalano S, et al. LSD1 inhibition disrupts super-enhancer-driven oncogenic transcriptional programs in castration-resistant prostate cancer. Cancer Res. 2023;83:1684–98.
Mandl A, Jasmine S, Krueger T, Kumar R, Coleman IM, Dalrymple SL et al. LSD1 inhibition suppresses ASCL1 and de-represses YAP1 to drive potent activity against neuroendocrine prostate cancer. bioRxivorg [Internet]. 2024; Available from: https://pubmed.ncbi.nlm.nih.gov/38328141/
Hollebecque A, Salvagni S, Plummer R, Niccoli P, Capdevila J, Curigliano G, et al. Clinical activity of CC-90011, an oral, potent, and reversible LSD1 inhibitor, in advanced malignancies. Cancer. 2022;128:3185–95.
Study Details. NCT05268666 [Internet]. [cited 2024 Nov 10]. Available from: https://clinicaltrials.gov/study/NCT05268666
van Mierlo G, Veenstra GJC, Vermeulen M, Marks H. The complexity of PRC2 subcomplexes. Trends Cell Biol. 2019;29:660–71.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9.
Venkadakrishnan VB, Presser AG, Singh R, Booker MA, Traphagen NA, Weng K, et al. Lineage-specific canonical and non-canonical activity of EZH2 in advanced prostate cancer subtypes. Nat Commun. 2024;15:6779.
Davies A, Nouruzi S, Ganguli D, Namekawa T, Thaper D, Linder S et al. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol [Internet]. 2021; Available from: https://doi.org/10.1038/s41556-021-00743-5
Choudhury AD, Xie W, Tewari A, Miyamoto DT, Kochupurakkal B, Ellis L, et al. A phase Ia/Ib study of talazoparib in combination with tazemetostat in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40:TPS195–195.
Schweizer MT, Penkov K, Choudhury AD, Calvo E, Frank RC, Liu L, et al. Phase 1 trial of mevrometostat (PF-06821497), a potent and selective inhibitor of enhancer of zeste homolog 2 (EZH2), in castration-resistant prostate cancer (CRPC). J Clin Oncol. 2024;42:5061–5061.
Daemen A, Yuen N, Wang A, Pankov A, Ulicna L, Chen C, et al. Abstract 6586: ORIC-944, a potent and selective allosteric PRC2 inhibitor with best-in-class properties, demonstrates combination synergy with AR pathway inhibitors in prostate cancer models. Cancer Res. 2024;84:6586–6586.
Study Details. NCT05413421 [Internet]. [cited 2024 Nov 11]; Available from: https://clinicaltrials.gov/study/NCT05413421
Li Y, He Y, Butler W, Xu L, Chang Y, Lei K et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci Transl Med [Internet]. 2019;11. Available from: https://doi.org/10.1126/scitranslmed.aax0428
Guo C, Sharp A, Gurel B, Crespo M, Figueiredo I, Jain S, et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature. 2023;623:1053–61.
Study Details. NCT03177187 [Internet]. [cited 2024 Nov 11]. Available from: https://clinicaltrials.gov/study/NCT03177187
Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95.
Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ, et al. A phase II trial of the Aurora kinase A inhibitor Alisertib for patients with castration-resistant and neuroendocrine prostate Cancer: efficacy and biomarkers. Clin Cancer Res. 2019;25:43–51.
Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen receptor pathway-independent prostate Cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–e4896.
Chan JM, Zaidi S, Love JR, Zhao JL, Setty M, Wadosky KM, et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science. 2022;377:1180–91.
Hanahan D. Hallmarks of Cancer: New dimensions. Cancer Discov. 2022;12:31–46.
Loh J-J, Ma S. Hallmarks of cancer stemness. Cell Stem Cell. 2024;31:617–39.
Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289–300.
Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386:437–48.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–905.
Kramer G, Shore ND, Joshua AM, Li XT, Poehlein CH, Schloss C, et al. KEYNOTE-365 cohorts E and F: phase 1b/2 study of pembrolizumab + lenvatinib combination therapy in patients with adenocarcinoma metastatic castration-resistant prostate cancer (mCRPC) or treatment-emergent neuroendocrine mCRPC (t-NE). J Clin Oncol. 2022;40:TPS215–215.
Augustin M, Laguerre B, Baldini C, Zedan AH, Gonzalez-Billalabeitia E, Fong PCC, et al. Pembrolizumab (pembro) plus lenvatinib (lenva) in patients (pts) with docetaxel-pretreated, metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort E. J Clin Oncol. 2024;42:148–148.
Agarwal N, Azad A, Carles J, Matsubara N, Oudard S, Saad F, et al. CONTACT-2: phase 3 study of cabozantinib (C) plus atezolizumab (A) vs second novel hormonal therapy (NHT) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Orthod. 2024;42:18–18.
Zaidi S, Park J, Chan JM, Roudier MP, Zhao JL, Gopalan A, et al. Single-cell analysis of treatment-resistant prostate cancer: implications of cell state changes for cell surface antigen-targeted therapies. Proc Natl Acad Sci U S A. 2024;121:e2322203121.
Ajkunic A, Sayar E, Roudier MP, Patel RA, Coleman IM, De Sarkar N, et al. Assessment of TROP2, CEACAM5 and DLL3 in metastatic prostate cancer: expression landscape and molecular correlates. NPJ Precis Oncol. 2024;8:104.
Pulido R, López JI, Nunes-Xavier CE. B7-H3: a robust target for immunotherapy in prostate cancer. Trends Cancer. 2024;10:584–7.
Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7-H3 as a therapeutic target in advanced prostate Cancer. Eur Urol. 2023;83:224–38.
Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–51.
Shi W, Wang Y, Zhao Y, Kim JJ, Li H, Meng C, et al. Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies. Sci Transl Med. 2023;15:eadf6724.
Yamada Y, Venkadakrishnan VB, Mizuno K, Bakht M, Ku S-Y, Garcia MM, et al. Targeting DNA methylation and B7-H3 in RB1-deficient and neuroendocrine prostate cancer. Sci Transl Med. 2023;15:eadf6732.
Shenderov E, De Marzo AM, Lotan TL, Wang H, Chan S, Lim SJ, et al. Neoadjuvant enoblituzumab in localized prostate cancer: a single-arm, phase 2 trial. Nat Med. 2023;29:888–97.
Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503.
Su Y, Liu Y, Behrens CR, Bidlingmaier S, Lee N-K, Aggarwal R et al. Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight [Internet]. 2018;3. Available from: https://doi.org/10.1172/jci.insight.121497
Shakhnazaryan N, Curry N, Rastogi M, Avins D, Pandey S, de Kouchkovsky I, et al. A phase 1b dose escalation study of FOR46, a novel antibody-drug conjugate targeting a tumor-specific epitope of CD46, in combination with enzalutamide (Enza) in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2024;42:5066–5066.
Bidkar AP, Wang S, Bobba KN, Chan E, Bidlingmaier S, Egusa EA, et al. Treatment of prostate cancer with CD46-targeted 225Ac alpha particle radioimmunotherapy. Clin Cancer Res. 2023;29:1916–28.
Wang S, Li J, Hua J, Su Y, Beckford-Vera DR, Zhao W, et al. Molecular imaging of prostate Cancer targeting CD46 using ImmunoPET. Clin Cancer Res. 2021;27:1305–15.
Study Details. NCT05245006 [Internet]. [cited 2024 Nov 10]. Available from: https://clinicaltrials.gov/study/NCT05245006
Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res. 2005;65:8809–17.
Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci U S A. 2018;115:E4473–82.
DeLucia DC, Cardillo TM, Ang L, Labrecque MP, Zhang A, Hopkins JE, et al. Regulation of CEACAM5 and therapeutic efficacy of an anti-CEACAM5–SN38 antibody–drug conjugate in neuroendocrine prostate cancer. Clin Cancer Res. 2021;27:759–74.
Study Details. NCT05204147 [Internet]. [cited 2024 Nov 10]; Available from: https://clinicaltrials.gov/study/NCT05204147
Imberti C, De Gregorio R, Korsen JA, Hoang TT, Khitrov S, Kalidindi T, et al. CEACAM5-targeted immuno-PET in androgen receptor-negative prostate cancer. J Nucl Med. 2024;65:1043–50.
Kusumi K, Sun ES, Kerrebrock AW, Bronson RT, Chi DC, Bulotsky MS, et al. The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat Genet. 1998;19:274–8.
Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7:302ra136.
Puca L, Gavyert K, Sailer V, Conteduca V, Dardenne E, Sigouros M et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med [Internet]. 2019;11. Available from: https://doi.org/10.1126/scitranslmed.aav0891
Ku S-Y, Wang Y, Garcia MM, Yamada Y, Mizuno K, Long MD et al. Notch signaling suppresses neuroendocrine differentiation and alters the immune microenvironment in advanced prostate cancer. J Clin Invest [Internet]. 2024 [cited 2024 Oct 11];134. Available from: https://pubmed.ncbi.nlm.nih.gov/39024561/
Mansfield AS, Hong DS, Hann CL, Farago AF, Beltran H, Waqar SN, et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3-expressing advanced solid tumors. NPJ Precis Oncol. 2021;5:74.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42–51.
Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–58.
Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage-SCLC: results from the phase 3 MERU study. J Thorac Oncol. 2021;16:1570–81.
Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, et al. AMG 757, a half-life extended, DLL3-Targeted bispecific T-Cell engager, shows high potency and sensitivity in Preclinical models of Small-Cell Lung Cancer. Clin Cancer Res. 2021;27:1526–37.
Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: an open-label, phase I study. J Clin Oncol. 2023;41:2893–903.
Ahn M-J, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med [Internet]. 2023; Available from: https://doi.org/10.1056/NEJMoa2307980
Dhillon S, Tarlatamab. First approval. Drugs. 2024;84:995–1003.
Aggarwal RR, Rottey S, Bernard-Tessier A, Mellado-Gonzalez B, Kosaka T, Stadler WM, et al. Phase 1b study of tarlatamab in de novo or treatment-emergent neuroendocrine prostate cancer (NEPC). J Clin Oncol. 2024;42:5012–5012.
Chou J, Egusa EA, Wang S, Badura ML, Lee F, Bidkar AP, et al. Immunotherapeutic targeting and PET imaging of DLL3 in small-cell neuroendocrine prostate Cancer. Cancer Res. 2023;83:301–15.
Korsen JA, Kalidindi TM, Khitrov S, Samuels ZV, Chakraborty G, Gutierrez JA et al. Molecular imaging of neuroendocrine prostate Cancer by targeting delta-like ligand 3. J Nucl Med [Internet]. 2022; Available from: https://doi.org/10.2967/jnumed.121.263221
Tendler S, Dunphy MP, Agee M, O’Donoghue J, Aly RG, Choudhury NJ et al. Imaging with [89Zr]Zr-DFO-SC16.56 anti-DLL3 antibody in patients with high-grade neuroendocrine tumours of the lung and prostate: a phase 1/2, first-in-human trial. Lancet Oncol [Internet]. 2024; Available from: https://doi.org/10.1016/S1470-2045(24)00249-3
Korsen JA, Gutierrez JA, Tully KM, Carter LM, Samuels ZV, Khitrov S, et al. Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer. Proc Natl Acad Sci U S A. 2022;119:e2203820119.
Study Details. NCT04471727 [Internet]. [cited 2024 Nov 10]; Available from: https://clinicaltrials.gov/study/NCT04471727
Study Details. NCT05652686 [Internet]. [cited 2024 Nov 10]; Available from: https://clinicaltrials.gov/study/NCT05652686
Butler W, Xu L, Zhou Y, Cheng Q, Hauck JS, He Y, et al. Oncofetal protein glypican-3 is a biomarker and critical regulator of function for neuroendocrine cells in prostate cancer. J Pathol. 2023;260:43–55.
Couzinet A, Suzuki T, Nakatsura T. Progress and challenges in glypican-3 targeting for hepatocellular carcinoma therapy. Expert Opin Ther Targets. 2024;1–15.
Funding
XZ received research funding from Conquer Cancer, the ASCO Foundation, the Prostate Cancer Foundation (PCF), and the Department of Defense (DOD) outside of this work. CKCD received research funding from the PCF outside of this work. RRA received research funding from the PCF, the DOD, and the National Cancer Institute outside of this work.
Author information
Authors and Affiliations
Contributions
X.Z. wrote the main manuscript text and prepared the figure and the table. R.R.A. oversaw the writing. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies with human subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Competing Interests
RRA is an advisor/consultant for AstraZeneca, Bayer, Bioexcel Therapeutics, Boxer Capital, Clovis, Curio, Dendreon, Exelixis, Janssen, Lumanity, Merck, Novartis, Pfizer, Prostate Cancer Clinical Trials Consortium, and Tersara Consulting; and received institutional research grants/funding from Amgen, AstraZeneca, Janssen, Merck, Novartis, and Zenith Epigenetics.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, X., Ding, CK.C. & Aggarwal, R.R. Emerging Therapeutic Targets of Neuroendocrine Prostate Cancer. Curr Oncol Rep 27, 362–374 (2025). https://doi.org/10.1007/s11912-025-01643-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-025-01643-9