Abstract
Purpose of Review
Head and neck tumors (HNC) rank sixth among cancers worldwide. Due to their late diagnosis and poor prognosis, they are a clinical challenge. However, recent years have seen a dynamic development of science on the microbiome. The aim of the study is to discuss the role of the microbiome in HNC, the impact of the microbiome on oncogenesis, the course of the disease, as well as on treatment, and its toxicity.
Recent Findings
The microbiome’s influence on oncogenesis, the course of the disease, and the effectiveness of oncological treatment have been confirmed in cancers of the colon, pancreas, lungs, and prostate. There is no solid literature on HNC. Many studies indicate disruption of the oral microbiome and periodontal disease as potential cancer risk factors. Disruption of the microbiome increases radiotherapy’s toxicity, intensifying radiation reactions.
Summary
The microbiome plays an important role in cancer. It is a new target in research into new therapies. It may also be a prognostic marker of cancer development. Changes in the composition of the microbiome modulate the effectiveness of oncological treatment. More research is needed on the microbiome and its effects on HNC.
Similar content being viewed by others
Introduction
The incidence of head and neck cancer (HNC) globally ranks sixth, with over half a million new patients annually [1]. Men suffer from HNC more often than women: 5.8/100,000 to 2.3/100,000. HNC is a heterogeneous group of neoplastic diseases with significant differences between the European and US populations in terms of incidence and survival [2, 3]. The 5-year survival rate in HNC is below 50% [4, 5]. The most common cancer among HNC is laryngeal cancer; the prevalence in the EURoCare-5 population was 4.6/100,000 [3]. Research shows that infectious agents cause 15 to 20% of cancers, 20 to 30% are caused by smoking, and 30 to 35% are caused by lifestyle, improper diet, lack of physical activity, and obesity [6]. The main risk factors for HNC with a well-known mechanism of carcinogenesis are smoking, alcohol consumption, and the human papillomavirus (HPV), primarily type 16, and the Epstein-Barr virus (EBV). Oncogenic viruses have been well studied and described. HPV-based HNCs have different clinical characteristics and a better prognosis [7]. EBV detected in nasopharyngeal neoplasm significantly worsens the prognosis [8, 9]. Oncogenic viruses integrate viral DNA into the host genome [10] and inactivate tumor suppressor genes like p53 [11]. Despite combined treatment methods based on surgery, radiotherapy, chemotherapy, and immunotherapy, 5-year survival in HNC varies between 25 and 60% [3].
The microbiome is all the micro-organisms found in a tissue, organ, or the entire body. It was first described in 2001 [12]. The microbiome includes genes and genomes of the microbiota, as well as products of the microflora and the host, such as plasmid DNA, viruses, archaea, and fungi [13]. The human microbiome is currently undergoing numerous analyses; its impact on the incidence of many diseases, including cancer, is being assessed. The human microbiome consists of over 100 billion organisms, mainly found on mucous membranes, giving 2 kg of mass [14, 15]. Due to the very important role it plays in the pathophysiology of human diseases, it has been called “the last human organ under active research” [16] and “the second brain” [17]. The human microbiome is individually variable; the greatest influence on its composition is exerted by environmental factors and the host organism [18, 19]. Smoking tobacco alters the lung microbiome [20]. A high-fat diet causes dysbiosis with the dominant Fusobacterium nucleatum, considered to be an oncogenic bacterium [21]. Moreover, drugs such as metformin and proton pump inhibitors alter the gastrointestinal microbiome [22]. The composition of the microbiome has been defined by the 16S ribosomal RNA (rRNA) gene for bacteria [23].
The microbiome imbalance is dysbiosis, which is usually associated with diseases. Dysbiosis is primarily associated with intestinal inflammation, obesity, and allergies. It also occurs in multiple sclerosis, autism, depression, as well as in cancer [24,25,26]. It is believed that the pathogenic microbiome promotes oncogenesis through mucositis and general disorder of the body’s metabolism. The impact of the microbiome on the immune system function is particularly important [27, 28]. The analysis of over 1500 tests showed that tumor microbiome is mainly intracellular bacteria [29]. Extracellular microorganisms that are a microflora of intestines, mouth, vagina, or skin also have a very important function [30,31,32], and also affect oncological treatment [33].
Study Design
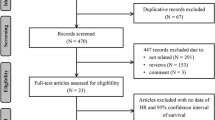
The purpose of the work is to discuss the role of the microbiome in HNC, the influence of the microbiome on oncogenesis, the course of the disease, as well as on treatment and its toxicity. The literature search strategy was carried out using the Web of Science and PubMed base based on the keyword combination: Head and Neck Cancer, Microbiome, Microbiota, Oncogenesis, Radiotherapy, Chemotherapy, Immunotherapy, Toxicity. Studies were excluded if any of the concepts included in the search were not central to the article or if they used culture-based methods.
Microbiome Analysis Technologies
Microbiome analysis technologies are based mainly on metagenomic bacterial sequencing of 16 s RRNA and shotgun sequencing. 16 s RRNA sequencing allows for the quantitative assessment of bacterial composition, assessing their type [34, 35]. Shotgun sequencing allows you to assess the composition and number of microorganisms [36]. The new technology is also IS-PRO, where the S-13 RRNA gene regions with lengths specific to each species of microorganism are amplified [37]. Analyzing MRNA, on the other hand, shows the activity of organisms, but only at the time of the study, it is not possible to determine the long-term relationships based on it, but only gene expression when the sample is taken [38]. Other techniques include the assessment of proteins or other metabolites, which include fatty acids, bile acids, and vitamins. Thanks to this technology, we are able to assess the effect of the microbiome on diseases. This opened the path to new diagnostic and treatment opportunities, giving hope for new anti-cancer therapies, especially in HNC, where treatment and survival of patients is unsatisfactory.
Influence of the Microbiome on Neoplastic Diseases
The first reports of the microbiome and oncogenic or pathogenic microbiome date back to the beginning of the twenty-first century. The intensive development of science concerning the microbiome allowed for the evaluation of its influence on cancerous diseases. Neoplastic tissue is characterized by intense inflammation; the disorder mainly affects the immune system. Uncontrolled inflammation leads to hypoxia and necrosis, which facilitates the growth of anaerobic bacteria [39]. Inflammation in neoplastic disease has been known for many years, especially in HNC and neoplasms of the gastrointestinal tract, where the rich flora of the upper respiratory and gastrointestinal tract allows for uncontrolled bacterial growth in a favorable environment.
The metabolites produced by the host organism and its microbiome are modulators of many processes, maintain homeostasis, maintain proper metabolism, stabilize inflammation, and proliferation [40•]. They can change the microenvironment of tissues, especially cancerous tissues. Bacteria activate the immune system, can increase the number of neutrophils, and upregulate oncogenic pathways. Bacteria accelerate neoplastic transformations in patients with genetic susceptibility to cancer [41]. First of all, the reduction in the diversity of bacterial species is worrying; the predominance of one or more pathogenic species may be the beginning of cancer development.
The above information allowed us to conclude that the tumor microbiome and the surrounding tissues differ significantly from the microbiome of healthy tissue. The microbiome in neoplastic diseases not only changes the flora of the tumor and the surrounding area, it can move through the blood along with neoplastic cells to distant organs and systems, similarly to distant metastases [42]. The oncogenic microbiome may cause dysregulation of the entire body’s metabolism. The same types of bacteria were found in the upper respiratory tract and the upper gastrointestinal tract as in esophageal neoplasms or cervical cancer, these relationships have not yet been elucidated, but this confirms the influence of the microbiome on all human tissues [43, 44].
The microbiome has the greatest influence on the mucous membranes and richly colonized tissues. The digestive system is home to the most bacteria. Helicobacter pylori, a bacterium recognized as oncogenic, contributes to the development of gastric cancer by increasing the expression of cyclooxygenase 2 (COX-2), cytokines, and reactive oxygen species, thus the bacterium induces DNA oxidative damage, promoting oncogenesis [45]. As a result, Helicobacter pylori causes about 5% of cancers in the world [46].
The advantage of pathogens such as Fusobacteria, Providencia, and Actinobacter in neoplastic tissue has been confirmed in colorectal neoplasms [47, 48]. Many scientific reports confirm the oncogenic effect of these bacteria on the mucosa; moreover, they increase staging and grading of the tumor and the response to chemotherapy is much worse [49, 50]. In their study, Bullman et al. [51] presented the effect of metronidazole on the reduction of the microbiome and inhibition of colon cancer growth. Intestinal dysbiosis also reduces the amount of protective butyrate and contributes to an increase in cell proliferation. Changes in the gut microbiome also affect other cancers, and a decreased response to immunotherapy has been observed in kidney cancer, melanoma, and lung cancer in people with dysbiosis. This is confirmed by the fact that the gut microbiome influences the immune system.
The microbiome in patients with lung cancer differs from that in patients with chronic lung disease, with Streptococcus and Prevotella dominating in cancer [44]. According to Lee et al. [52], Veillonella and Megasphaera can be considered as biomarkers for lung cancer. In pancreatic cancer, the microbiome is the same as in the intestines—it is suspected that the microbiome may change the pancreatic microenvironment, leading to oncogenesis [53]. Changes in the microbiome have also been confirmed in breast cancer, where the bacteria present in the cancer are different from those found in normal tissue [54]. Proteobacteria, Actinobacteria, and Firmicutes are found in breast cancer and the surrounding tissue. Brewster et al. [55] found microbiome changes in female genitals that may be associated with ovarian cancer and endometriosis. In prostate cancer, the presence of pathogenic bacteria such as Escherichia, Mycoplasma, and Cutibacterium has been confirmed [56]. Studies on mice have shown that implantation of Escherichia coli and Cutibacterium acnes after prostatectomy induced inflammation and prostate adenocarcinoma [57.58]. In bladder cancer, the amount of Fusobacterium and Campylobacter is increased [59]. In the described examples, it was confirmed that the neoplastic tissue has a modified microbiome that can be considered pathological.
Influence of the Microbiome on Head and Neck Cancers
Many cancers arise from inflammation, and it has been confirmed by HNC that smoking and alcohol consumption induce inflammation that leads to cancer formation. Research has confirmed that the microbiome can also promote inflammation and oncogenesis. The microbiome promotes inflammation in the airway epithelium in chronic obstructive pulmonary disease, cystic fibrosis, and asthma [60]. The interaction of the microbiome is synergistic with that of alcohol; bacteria metabolize ethanol, mediating the formation of acetaldehyde, which is a highly toxic compound. Acetaldehyde interferes with DNA synthesis and repair, increasing the risk of HNC [61]. Bacteria present in saliva—Streptococcus salivarius, Corynebacterium, and Stomatococcus have strong oxidizing properties, which allows for intensive metabolism of ethanol [62]. The tissue adjacent to the tumor may have the most significant impact on oncogenesis—its dysbiosis causes changes in the functioning of the immune system, which makes it possible to oncogenesis. Most of the literature is about the oral microbiome, which is the richest in the entire human body. There are 770 species of microorganisms in the oral cavity. Their composition is influenced by hygiene, smoking, diet, and alcohol consumption [63, 64]. Mukherjee et al. [65], Yost et al. [66], and Yang et al. [67] noted that in patients with oral cancer, dysbiosis enhances tumor growth. Porphyromonas gingivalis has the ability to stimulate oncogenesis in the oral cavity [68]; high levels of class G antibodies in the serum against this bacterium found in patients with gastrointestinal cancer and HNC [69]. Patients with diagnosed Porphyromonas gingivalis in the oral cavity have higher mortality [69]. This bacterium stimulates the production of myeloid-derived dendritic suppressor cells, which inhibit cytotoxic T lymphocytes, also induce overexpression of pro-matrix metalloproteinase-9 and reduce TP53 expression, thus inducing cell proliferation [70, 71].
The increase in mortality due to HNC is also significant with accompanying periodontitis [72]. Streptococcus anginosus in dental plaque increases the synthesis of nitric oxide and cyclooxygenase-2, increasing the risk of DNA damage [73]. However, no such correlation was observed in caries. Gong et al. [74] found the advantage of Fusobacterium in cancer of the larynx and surrounding tissue. The microbiome of the upper respiratory tract also affects the lower respiratory tract; dysbiosis in this area contributes to the development of lung cancer—the presence of the same pathogenic bacteria in the oral cavity and lung tumor tissues in patients with lung cancer has been confirmed [75]. On the surface of oral carcinomas, anaerobes such as Actinomyces, Clostridium, Fusobacterium, Porphyromonas, and Bacteroides are found. Candida albicans is the dominant among fungi, as well as aerobic bacteria such as Klebsiella, Citrobacter, Streptococcus, Enterobacter, and Serratia [76]. Frank et al. [77••] confirmed that Lactobacillus abundance is increased and Neisseria decreased in HNC. They also confirmed that reducing the pathogenic microbiome inhibits HNC oncogenesis, while transfer of microflora from HNC mice accelerates oncogenesis. It has been suggested that the administration of antibiotics prevents or delays the induction of the Ahr pathway [78]. AhR activation is a very important pathway in neoplastic diseases [78]. On the other hand, Dou et al. [79•] observed that increased numbers of Schlegelella and Methyloversatilis in HNC are associated with poor prognosis, while the dominant Bacillus, Lactobacillus, and Sphingomonas are found in patients with favorable prognosis. The oncogenic effect of Fusobacterium nucleatum in oral cancer has also been confirmed; it interacts directly with epithelial cells, causing faster growth and easier spread of the tumor [80]. This bacterium also inhibits apoptosis, thereby stopping the attempt to eliminate damaged cells. Little is found in the literature on the correlation of the microbiome with laryngeal cancer. Gong et al. [74] confirmed significant differences in the microbiome and recognized bacteria as a potential carcinogen. Haemophilus influenzae may also promote oncogenesis by IL-17 and neutrophil infiltration [81].
HPV-positive HNC have different clinical characteristics than other cancers; they occur in younger, not smoking, not drinking alcohol patients; their prognosis is better; and they are usually detected at an early stage [82]. Oncogenic bacteria can also change the course of cancer; however, to indicate these relationships, further scientific research is needed.
The microbiome can also support the fight against cancer or prevent them, Corynebacterium and Kingella change the risk of HNC by biodegradation and metabolism of poisonous substances such as toluene, styrene, and chlorobenzene. Actinomyces can reduce the risk of throat cancer, while Neisseria sicca can reduce the risk of oral cancer.
The Role of the Microbiome in Cancer Therapy
The microbiome can influence the effectiveness of immunotherapy, chemotherapy, and radiation therapy. In patients with metastatic melanoma treated with anti-CTLA4 ipilimumab, microflora enriched with Firmicutes and Faecalibacterium gives longer progression-free and overall survival than in microbiota dominated by Bacteroides [83]. Bifidobacterium also supports melanoma therapy [84]. According to Routy et al. [85], patients resistant to immunotherapy are mainly people with dysbiosis and those who have undergone antibiotic therapy. Patients immediately after antibiotic therapy have a greater risk of rapid disease progression in lung cancer [86].
The microbiome also influences the effectiveness of cisplatin and cyclophosphamide chemotherapy [87, 88]. Modifying the microbiome, reducing pathogenic bacteria supports the effectiveness of immunotherapy and chemotherapy, and reduces the side effects of this treatment.
Radiotherapy is a very important method in the treatment of HNC. Radiotherapy can be used as a radical treatment or in combination with chemotherapy as adjuvant or palliative treatment [89•]. The effect of radiation therapy is to damage cancer cells, but also to damage the cells surrounding the cancer. Radiotherapy causes side effects such as stomatitis, xerostomia, dysphagia, odynophagia, and chronic sinusitis [89•, 90, 91]. These symptoms have a significant impact on the quality of life of patients, and sometimes even lead to the discontinuation of oncological treatment. Intensive research is aimed at reducing the toxicity of radiotherapy treatment. One potential approach is to modify the microbiome to protect the mucous membranes. The radiation induces inflammatory processes, leading to apoptosis or cell death. Necrotic tissue is formed at the site of intense radiation, which promotes dysbiosis and uncontrolled multiplication of bacteria. Microbiome homeostasis during radiotherapy depends on the number of bacteriophages, the number of which also declines due to radiation. Commensal bacteria can become pathogenic, and the growth of pathogenic bacteria can lead to serious inflammatory complications. Stokman et al. [92] assessed the effect of topical broad-spectrum antibiotics on the microbiome and the severity of stomatitis after radiotherapy and found that it had no effect and did not alleviate the symptoms of the disease. Antibiotic therapy may have a negative effect on the dysbiosis during radiotherapy. Studies have confirmed that the diversity of the microbiome decreases with the radiation dose rate [93, 94]. The lower diversity of bacteria causes dysbiosis and the uncontrolled multiplication of pathogenic bacteria.
Over 90% of patients with HNC develop stomatitis after radiotherapy, and 60% of them have inflammation classified as severe accompanied by complete dysphagia [90]. Dysbiosis promotes the persistence of ulcers and delays healing [95•]. Actinobacillus, Mannheimia, and Streptobacillus are associated with increased severity of oral mucositis [96]. Fusobacterium and Haemophilus dominant in the oral microbiome before radiotherapy are associated with susceptibility to inflammatory complications. Prevotella, Fusobacterium, and Streptococcus are considered as prognostic biomarkers of the onset of oral mucositis, while Megasphaera and Cardiobacterium are biomarkers of severe inflammation [95•]. In a study by Jiang et al. [97], it was shown that patients who received probiotics during chemoradiotherapy developed less oral mucositis compared to the group without probiotics in therapy (15.5% vs. 45.7%). A study by Ma et al. [98] showed that patients with probiotic therapy more often undergo radiotherapy without complications, compared to the group without probiotics in therapy, where patients discontinued treatment due to complication. Late complications of radiotherapy include tumors induced by oncological treatment, especially after HNC treatment, one of the causes may be persistent dysbiosis of the upper respiratory tract.
The microbiome can be modified by symbiotic treatment, using prebiotics and probiotics. Bifidobacterium improves the course of diabetes and allergies. According to Rong et al. [99], the intake of Lactobacillus helveticus may suppress hyperplasia and oncogenesis by reducing the number of T lymphocytes. In patients with dysbiosis and lack of improvement of flora with symbiotic treatment, the use of antibiotics may also be effective. Maintaining a normal microbiome in the body is a way to avoid cancer. In a study conducted on mice, probiotics used in inhalation strengthened lung resistance to metastases [100].
Limitations of the Study
Many publications are based on small study groups. The microbiome is influenced by many environmental factors that should be thoroughly assessed. Due to the ongoing research on the microbiome, we should be very critical of knowledge on this subject and select only publications with a large study group and an appropriate control group.
Conclusions
HNC is a multifactorial disease; environmental factors influence its development, course, and treatment.
The differences between the microbiome of healthy people and HNC patients have been confirmed.
The microbiome can be oncogenic by intensifying the inflammation of the mucous membranes, systemic effects, as well as changes in anti-cancer resistance or by inhibiting the effectiveness of cancer therapy.
The presence of HNC drives changes in the microbiome or dysbiosis induces oncogenesis.
The upper respiratory tract dysbiosis with smoking and alcohol consumption is an important HNC risk factor.
Bacteria can be a prognostic biomarker of HNC development.
The interaction of the dysbiosis with immunotherapy, chemotherapy, and radiotherapy affects the effectiveness of treatment and the intensification of the side effects of these therapies.
Many clinical trials about the microbiome and dysbiosis are still at an early stage, but there is enormous potential to use it in the future.
A targeted therapy against the microbiome of cancer can contribute to HNC prevention, inhibiting cancer progression or increasing the effectiveness of treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma – an update. CA Cancer J Clin. 2015;65:401–21.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Gatta G, Botta L, Sánchez MJ, Anderson LA, Pierannunzio D, Licitra L. EUROCARE Working Group: prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer. 2015;51(15):2130–43.
Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:873–98.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72.
Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Ma L, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Wang J, Zhan Q. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–5.
Lydiatt WM, Patel SG, Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers major changes in the american joint committee on cancer eighth edition cancer staging manual head and neck cancers major edition changes. Cancer J Clin. 2017;67(2):122–37.
Liu TB, Zheng ZH, Pan J, Pan LL, Chen LH. Prognostic role of plasma Epstein-Barr virus DNA load for nasopharyngeal carcinoma: a meta-analysis. Clin Invest Med. 2017;40(1):E1-12.
Alfieri S, Iacovelli NA, Marceglia S, Lasorsa I, Resteghini C, Taverna F, et al. Circulating pre-treatment Epstein-Barr virus DNA as prognostic factor in locally advanced nasopharyngeal cancer in a non-endemic area. Oncotarget. 2017;8(29):47780–9.
Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98.
Tornesello ML, Annunziata C, Tornesello AL, Buonaguro L, Buonaguro FM. Human oncoviruses and p53 tumor suppressor pathway deregulation at the origin of human cancers. Cancers (Basel). 2018;10(7):213.
Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract-a role beyond infection. Nat Rev Urol. 2015;12(2):81–90.
Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31.
McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, DeRight Goldasich L, Dorrestein PC, Dunn RR, Fahimipour AK, Gaffney J, Gilbert JA, Gogul G, Green JL, Hugenholtz P, Humphrey G, Huttenhower C, Jackson MA, Janssen S, Jeste DV, Jiang L, Kelley ST, Knights D, Kosciolek T, Ladau J, Leach J, Marotz C, Meleshko D, Melnik AV, Metcalf JL, Mohimani H, Montassier E, Navas-Molina J, Nguyen TT, Peddada S, Pevzner P, Pollard KS, Rahnavard G, Robbins-Pianka A, Sangwan N, Shorenstein J, Smarr L, Song SJ, Spector T, Swafford AD, Thackray VG, Thompson LR, Tripathi A, Vázquez-Baeza Y, Vrbanac A, Wischmeyer P, Wolfe E, Zhu Q, Knight R, American Gut Consortium. American Gut: an open platform for citizen science microbiome research. Systems. 2018;3(3):00031–18.
Flint HJ. The impact of nutrition on the human microbiome. Nutr Rev. 2012;70(Suppl 1):S10–3.
Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18(Suppl 4):2–4.
Ochoa-Repáraz J, Kasper LH. The second brain: is the gut microbiota a link between obesity and central nervous system disorders? Curr Obes Rep. 2016;5(1):51–64.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5.
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177.
Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, von Muhlinen N, Polley EC, Bowman ED, Khan MA, Robles AI, Cooks T, Ryan BM, Padgett N, Dzutsev AH, Trinchieri G, Pineda MA, Bilke S, Meltzer PS, Hokenstad AN, Stickrod TM, Walther-Antonio MR, Earl JP, Mell JC, Krol JE, Balashov SV, Bhat AS, Ehrlich GD, Valm A, Deming C, Conlan S, Oh J, Segre JA, Harris CC. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018;19(1):123.
O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706.
Buhmann MT, Abt D, Nolte O, Neu TR, Strempel S, Albrich WC, Betschart P, Zumstein V, Neels A, Maniura-Weber K, Ren Q. Encrustations on ureteral stents from patients without urinary tract infection reveal distinct urotypes and a low bacterial load. Microbiome. 2019;7(1):60.
Human Microbiome Project C. A framework for human microbiome research. Nature. 2012;486(7402):215–21.
Forbes JD, Van Domselaar G, Bernstein CN. Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflamm Bowel Dis. 2016;22(4):817–25.
Mowry EM, Glenn JD. The dynamics of the gut microbiome in multiple sclerosis in relation to disease. Neurol Clin. 2018;36(1):185–96.
Rodriguez M, Wootla B, Anderson G. Multiple sclerosis, gut microbiota and permeability: role of tryptophan catabolites, depression and the driving down of local melatonin. Curr Pharm Des. 2016;22(40):6134–41.
Mitsuhashi A, Okuma Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin Transl Oncol. 2018;20(8):966–74.
Floch P, Mégraud F, Lehours P. Helicobacter pylori strains and gastric MALT Lymphoma. Toxins (Basel). 2017;9(4):132. https://doi.org/10.3390/toxins9040132.
Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–80.
Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271ps.
Gaonkar PP, Patankar SR, Tripathi N, Sridharan G. Oral bacterial flora and oral cancer: the possible link? J Oral Maxillofac Pathol. 2018;22(2):234–8.
Kyrgiou M, Mitra A, Moscicki AB. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168–82.
Qiu Q, Lin Y, Ma Y, Li X, Liang J, Chen Z, et al. Exploring the emerging role of the gut microbiota and tumor microenvironment in cancer immunotherapy. Front Immunol. 2020;11:612202.
Wang Y, Qian PY. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE. 2009;4(10):e7401.
Pei AY, Oberdorf WE, Nossa CW, Agarwal A, Chokshi P, Gerz EA, Jin Z, Lee P, Yang L, Poles M, Brown SM, Sotero S, Desantis T, Brodie E, Nelson K, Pei Z. Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl Environ Microbiol. 2010;76(12):3886–97.
Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35(9):833–44. https://doi.org/10.1038/nbt.3935.Erratum.In:NatBiotechnol.2017Dec8;35(12):1211.
Budding AE, Hoogewerf M, Vandenbroucke-Grauls CM, Savelkoul PH. Automated broad-range molecular detection of bacteria in clinical samples. J Clin Microbiol. 2016;54(4):934–43.
Franzosa EA, Morgan XC, Segata N, Waldron L, Reyes J, Earl AM, Giannoukos G, Boylan MR, Ciulla D, Gevers D, Izard J, Garrett WS, Chan AT, Huttenhower C. Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci U S A. 2014;111(22):E2329–38.
Baban CK, Cronin M, O’Hanlon D, O’Sullivan GC, Tangney M. Bacteria as vectors for gene therapy of cancer. Bioeng Bugs. 2010;1(6):385–94.
• Picardo SL, Coburn B, Hansen AR. The microbiome and cancer for clinicians. Crit Rev Oncol Hematol. 2019;141:1–12. Review paper on the role of the microbiome in cancer and its therapy.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79.
Syed Khaja AS, Toor SM, El Salhat H, Faour I, Ul Haq N, Ali BR, Elkord E. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8(20):33159–71.
Lam KC, Vyshenska D, Hu J, Rodrigues RR, Nilsen A, Zielke RA, Brown NS, Aarnes EK, Sikora AE, Shulzhenko N, Lyng H, Morgun A. Transkingdom network reveals bacterial players associated with cervical cancer gene expression program. PeerJ. 2018;19(6):e5590.
Liu Y, Lin Z, Lin Y, Chen Y, Peng XE, He F, Liu S, Yan S, Huang L, Lu W, Xiang Z, Hu Z. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J Med Microbiol. 2018;67(8):1058–68.
Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248(3):171–83.
de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–90. https://doi.org/10.1016/S2214-109X(19)30488-7.
Gao R, Kong C, Huang L, Li H, Qu X, Liu Z, Lan P, Wang J, Qin H. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(11):2073–83.
Burns MB, Lynch J, Starr TK, Knights D, Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med. 2015;7(1):55.
Lee DW, Han SW, Kang JK, Bae JM, Kim HP, Won JK, Jeong SY, Park KJ, Kang GH, Kim TY. Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann Surg Oncol. 2018;25(11):3389–95.
Koi M, Okita Y, Carethers JM. Fusobacterium nucleatum infection in colorectal cancer: linking inflammation, DNA mismatch repair and genetic and epigenetic alterations. J Anus Rectum Colon. 2018;2(2):37–46.
Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Cajal SRY, Fasani R, Aguirre AJ, NG K, Elez E, Ogino S, Tabernero J, Fuchs CS, Hahn WC, Nuciforo P, Meyerson M. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–8.
Lee SH, Sung JY, Yong D, Chun J, Kim SY, Song JH, Chung KS, Kim EY, Jung JY, Kang YA, Kim YS, Kim SK, Chang J, Park MS. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89–95.
Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–16.
Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, Zhang L. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front Oncol. 2018;17(8):318.
Brewster WR, Ko EM, Keku TO. An evaluation of the microbiota of the upper genital tract of women with benign changes and epithelial ovarian. Cancer. 2016;34(15_suppl):5568.
Yow MA, Tabrizi SN, Severi G, Bolton DM, Pedersen J, BioResource APC, Giles GG, Southey MC. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect Agent Cancer. 2017;13(12):4.
Shinohara DB, Vaghasia AM, Yu SH, Mak TN, Brüggemann H, Nelson WG, De Marzo AM, Yegnasubramanian S, Sfanos KS. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate. 2013;73(9):1007–15.
Simons BW, Durham NM, Bruno TC, Grosso JF, Schaeffer AJ, Ross AE, Hurley PJ, Berman DM, Drake CG, Thumbikat P, Schaeffer EM. A human prostatic bacterial isolate alters the prostatic microenvironment and accelerates prostate cancer progression. J Pathol. 2015;235(3):478–89.
Bučević Popović V, Šitum M, Chow CT, Chan LS, Roje B, Terzić J. The urinary microbiome associated with bladder cancer. Sci Rep. 2018;8(1):12157.
Mao Q, Jiang F, Yin R, Wang J, Xia W, Dong G, Ma W, Yang Y, Xu L, Hu J. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018;28(415):40–8.
Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–12.
Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int J Cancer. 2004;111(4):480–3.
An X, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Freedman ND, Alekseyenko AV, Wu J, Yang L, Pei Z, Hayes RB, Ahn J. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6(1):59.
Hsiao JR, Chang CC, Lee WT, Huang CC, Ou CY, Tsai ST, Chen KC, Huang JS, Wong TY, Lai YH, Wu YH, Hsueh WT, Wu SY, Yen CJ, Chang JY, Lin CL, Weng YL, Yang HC, Chen YS, Chang JS. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogenesis. 2018;39(6):778–87.
Mukherjee PK, Wang H, Retuerto M, Zhang H, Burkey B, Ghannoum MA, Eng C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget. 2017;8(57):97273–89.
Yost S, Stashenko P, Choi Y, Kukuruzinska M, Genco CA, Salama A, Weinberg EO, Kramer CD, Frias-Lopez J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 2018;10(4):32.
Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW, Liu H, Huang PJ, Hu SN, Liao CT, Chang KP, Chang YL. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;3(9):862.
Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–15.
Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–8.
Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat. 2019;18:1533033819867354.
Armitage GC. Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontol. 2000;2010(53):70–88.
Katarkar A, Saha A, Mukherjee S, Kundu D, Bandyopadhyay P, Chaudhuri K. Telomerase expression in individuals with chronic and aggressive periodontitis. J Periodontol. 2015;86:656–65.
Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, Harada R, Kimura S. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005;11:151–6.
Gong H, Shi Y, Xiao X, Cao P, Wu C, Tao L, Hou D, Wang Y, Zhou L. Alterations of microbiota structure in the larynx relevant to laryngeal carcinoma. Sci Rep. 2017;7(1):5507.
Hasegawa A, Sato T, Hoshikawa Y, Ishida N, Tanda N, Kawamura Y, Kondo T, Takahashi N. Detection and identification of oral anaerobes in intraoperative bronchial fluids of patients with pulmonary carcinoma. Microbiol Immunol. 2014;58(7):375–81.
Hooper SJ, Crean SJ, Fardy MJ, Lewis MA, Spratt DA, Wade WG, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56(12):1651–9.
•• Frank DN, Qiu Y, Cao Y, Zhang S, Lu L, Kofonow JM, Robertson CE, Liu Y, Wang H, Levens CL, Kuhn KA, Song J, Ramakrishnan VR, Lu SL. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene. 2022;41(9):1269–80. https://doi.org/10.1038/s41388-021-02137-1. The work describes the differences in the microbiome in patients with HNC in relation to the control group and its influence on the treatment of cancer.
Quintana FJ. The aryl hydrocarbon receptor: a molecular pathway for the environmental control of the immune response. Immunology. 2013;138:183–9.
• Dou Y, Ma C, Wang K, Liu S, Sun J, Tan W, Neckenig M, Wang Q, Dong Z, Gao W, Chen A, Zhang D, Wang B, Shi L, Nan Z, Ai D, Yu W, Liu J, Song B, Zhao L, Shao Q, Zhu Y, Wang T, Wang J, Hu W, Wei F, Xu X, Qu X. Dysbiotic tumor microbiota associates with head and neck squamous cell carcinoma outcomes. Oral Oncol. 2022;124:105657. https://doi.org/10.1016/j.oraloncology.2021.105657. Work that describes the influence of the microbiome on prognosis in patients with HNC.
Gallimidi AB, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, Nussbaum G, Elkin M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613–23.
Jungnickel C, Schmidt LH, Bittigkoffer L, Wolf L, Wolf A, Ritzmann F, Kamyschnikow A, Herr C, Menger MD, Spieker T, Wiewrodt R, Bals R, Beisswenger C. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene. 2017;36(29):4182–90.
Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4(1):58.
Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, Vaysse T, Marthey L, Eggermont A, Asvatourian V, Lanoy E, Mateus C, Robert C, Carbonnel F. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, Rouche JA, Zitvogel L, Zalcman G, Albiges L, Escudier B, Routy B. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–44.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
• Kumpitsch C, Moissl-Eichinger C, Pock J, Thurnher D, Wolf A. Preliminary insights into the impact of primary radiochemotherapy on the salivary microbiome in head and neck squamous cell carcinoma. Sci Rep. 2020;10(1):16582. Work that evaluates the effects of radiochemotherapy on the microbiome in patients with HNC.
Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front. Oncol. 2017;7:89. https://doi.org/10.3389/fonc.2017.00089.
Feng S, Fan Y, Liang Z, Yang G, Liao Z, Guo L. Effect of intranasal steroids on rhinosinusitis after radiotherapy for nasopharyngeal carcinoma: clinical study. J Laryngol Otol. 2016;130(3):265–71. https://doi.org/10.1017/S0022215115003448.
Stokman MA, Spijkervet FKL, Burlage FR, Dijkstra PU, Manson WL, de Vries EGE, et al. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: a double-blind randomised clinical trial. Br J Cancer. 2003;88(7):1012–6.
Gao L, Hu Y, Wang Y, Jiang W, He Z, Zhu C, et al. Exploring the variation of oral microbiota in supragingival plaque during and after head-and-neck radiotherapy using pyrosequencing. Arch Oral Biol. 2015;60(9):1222–30.
Hu Y-J, Wang Q, Jiang Y-T, Ma R, Xia W-W, Tang Z-S, et al. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS ONE. 2013;8(2):e56343.
• Reyes-Gibby CC, Wang J, Zhang L, Peterson CB, Do K-A, Jenq RR, et al. Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer. 2020;126(23):5124–36. A study that assesses the influence of the microbiome on the occurrence of oral mucositis as a complication of radiotherapy.
Zhu X-X, Yang X-J, Chao Y-L, Zheng H-M, Sheng H-F, Liu H-Y, et al. The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine. 2017;18(101647039):23–31.
Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081–90.
Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F. Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol. 2019;10:1050.
Rong J, Liu S, Hu C, Liu C. Single probiotic supplement suppresses colitis-associated colorectal tumorigenesis by modulating inflammatory development and microbial homeostasis. J Gastroenterol Hepatol. 2019;34(7):1182–92.
Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, Storti C, Triulzi T, Castelli C, Balsari A, Tagliabue E, Sfondrini L. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018;24(13):3528–38.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Head and Neck Cancers
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dorobisz, K., Dorobisz, T. & Zatoński, T. The Microbiome’s Influence on Head and Neck Cancers. Curr Oncol Rep 25, 163–171 (2023). https://doi.org/10.1007/s11912-022-01352-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01352-7