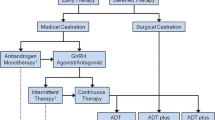
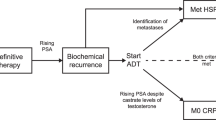
Abstract
In the past, the treatment options for patients with metastatic prostate cancer that progressed despite castrate levels of testosterone was limited, and no therapies provided an improvement in survival. The majority of these patients had extensive osseous disease, multiple comorbidities, and poor performance status. With the widespread use of prostate-specific antigen (PSA) to monitor their clinical course, patients have presented with less extensive disease and a better performance status. Clinical trial methodology has improved as well, through incorporation of post-therapy changes in PSA to evaluate novel agents. This approach allows more patients to enter clinical trials, and the results show that the majority of these patients will have significant reduction in pain, regression of measurable disease, and suppression of PSA. These data suggest that prostate cancer is not as resistant to chemotherapy as it was once thought to be.
Similar content being viewed by others
References
Newling DWW: Orchiectomy versus zoladex and flutamide in the treatment of newly diagnosed metastatic prostate cancer.Cancer 72:3793–3798, 1993.
Kyprianou N, English HF, Issacs JT: Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res 1990, 50:3748–3753.
Agus DB, Cordon-Cardo C, Fox W, et al.: Prostate cancer cell cycle regulators: response to androgen withdrawal and androgen independence. J Natl Cancer Inst 1999, 91:1869–1876. This human model of prostate cancer suggests that androgen withdrawal results in cell cycle arrest rather than apoptosis, and that release from the cell cycle arrest results in androgenindependent disease.
Yagoda A, Petrylak DP: Cytotoxic chemotherapy for advanced hormone resistant prostate cancer. Cancer 1993, 71(suppl 3):1098–1099.
Moore MJ, Osoba D, Murphy D, et al.: Use of palliative end points to evaluate the effects of mitoxantrone and low-dose prednisone in patients with hormonally resistant prostate cancer. J Clin Oncol 1994, 12:689–694, 1994.
Kelly WK, Scher HI, Mazumdar M, et al.: Prostate specific antigen as a measure of disease outcome in hormonerefractory prostatic cancer. J Clin Oncol 1993, 11:607–615.
Vollmer RT, Dawson NA, Vogelzang NJ: The dynamics of prostate specific antigen in hormone refractory prostate carcinoma. Cancer 1998, 83:1989–1994. The average relative PSA velocity is identified here as a prognostic factor in the treatment of hormone refractory prostate cancer.
Vollmer RT, Kantoff PW, Dawson NA, et al.: A prognostic score for hormone refractory prostate cancer: analysis of two Cancer and Leukemia Group B studies. Clin Cancer Res 1999, 5:831–837.
Bubley GJ, Carducci M, Dahut W, et al.: Eligibility and response guidelines for phase II clinical trials in androgenindependent prostate cancer: recommendations from the Prostate-specific Antigen Working Group. J Clin Oncol 1999, 17:3461–3467. Report on the consensus conference that established guidelines for documentation of phase II trials in patients with androgenindependent disease.
Scher HI, Mazumdar M, Kelly WK: Clinical trials in relapsed prostate cancer: defining the target. J Natl Cancer Inst 1996, 88:1623–1634.
Imbriaco M, Larson SM, Yeung HW, et al.: A new parameter for measuring metastatic bone involvement by prostate cancer: the bone scan index. Clin Cancer Res 1998, 4:1765–1772. These authors report the development of a novel method to analyze and quantitate changes on radionucleotide bone scans.
Sabbatini P, Larson SM, Kremer A, et al.: Prognostic significance of extent of disease in bone in patients with androgen independent prostate cancer. J Clin Oncol 1999, 17:948–957.
Tannock IF, Osoba D, Stockler MR, et al.: Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996, 14:1756–1764.
Kantoff PW, Halabi S, Conaway M, et al.: Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 Study. J Clin Oncol 1999, 17:2506–2513. Report of patients treated with mitoxantrone/hydrocortisone combination versus hydrocortisone alone. Delayed time to treatment failure was noted for the combination, but no difference in overall survival was observed.
Gunnarsson PO, Forshell GP, Fritjofsson A, et al.: Plasma concentrations of estramustine phosphate and its major metabolites in patients with prostatic carcinoma treated with different doses of estramustine phosphate (estracyt). Scand J Urol Nephrol 1981, 15:201–206.
Speicher LA, Barone L, Tew KD: Differential expression of cytoskeletal proteins in estramustine resistant human prostate carcinoma cell lines [abstract]. Proc ASCO 1993, 12:34.
Wang M, Stearns ME: Blocking of collagenase secretion by estramustine during in vitro tumor cell invasion. Cancer Res 1988, 48:6262–6271.
Seidman AD, Scher HI, Petrylak D, et al.: Estramustine and Vinblastine: use of prostate specific antigen as a clinical trial end point for hormone refractory prostatic cancer. J Urol 1994, 147:931–934.
Hudes GR, Greenberg R, Krigel RL, et al.: Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol 1992, 10:1754–1761.
Amato R, Logothetis C, Sella A, et al.: Preliminary results of a phase II trial of estramustine (EMCYT), vinblastine (VLB) and mitomycin-C (MMC) for patients with progressive androgen independent prostate carcinoma (AIPCa) [abstract]. Proc ASCO 1993, 12:34.
Hudes G, Einhorn L, Ross E, et al.: Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormone refractory prostate cancer: a Hoosier Oncology Group and Fox Chase network phase III trial. J Clin Oncol 1999, 17:3160–3166. Treatment with estramustine and vinblastine resulted in more patients achieving at least 50% post-therapy decline in PSA and longer time to progression of disease than treatment with vinblastine alone, according to this report. No difference in overall survival was noted.
Ellerhorst JA, Tu S-M, Amato RJ, et al.: Phase II trial of alternating weekly chemohormonal therapy for patients with androgen-indpendent prostate cancer. Clin Cancer Res 1997, 3:2371–2376.
Roth BJ, Yeap BY, Wilding G, et al.: Taxol in advanced hormone refractory carcinoma of the prostate: a phase II trial of the Eastern Cooperative Oncology Group. Cancer 1993, 72:2457–2460.
Speicher LA, Barone L, Tew KD: Combined antimicrotubule activity of estramustine and taxol in human prostatic carcinoma cell lines. Cancer Res 1992, 52:4433–4440.
Hudes GR, Nathan F, Khater C, et al.: Phase II trial of 96-hour paclitaxel plus oral estramustine in metastatic hormone refractory prostate cancer. J Clin Oncol 1997, 15:3156–3163.
Smith DC, Esper P, Strawderman M, et al.: Phase II trial of oral estramustine, oral etoposide and intravenous paclitaxel in hormone refractory prostate cancer. J Clin Oncol 1999, 17:1664–1671. The combination of paclitaxel, estramustine, and etoposide is active in patients with hormone-refractory prostate cancer. Forty-five percent of the patients in this reported study showed measurable disease regression, and over 50% post-therapy decline in PSA was observed in 65% of patients.
Kelly K, Gaudin P, Slovin S, et al.: Paclitaxel, estramustine and carboplatin in patients with advanced prostate cancer. In In Proceedings of the American Urological Association. Dallas, TX; 1999:77.
Petrylak DP, MacArthur RB, O’Connor J, et al.: Phase I trial of docetaxel with estramustine in androgen independent prostate cancer. J Clin Oncol 1999, 17:958–967. This study showed that the combination of estramustine and docetaxel is active in patients with hormone-refractory prostate cancer. Sixty-three percent of the patients showed more than 50% posttherapy decline in PSA, and the median overall survival for this group was 22.8 months.
Kreis W, Budman DR, Fetten J, et al.: Phase I of the combination of daily estramustine phosphate and intermittent docetaxel in patients with metastatic hormone refractory prostate cancer. Ann Oncol 1999, 10:33–38.
Savarese D, Taplin M, Marchesani B, et al.: A phase II study of docetaxel, estramustine, and low dose hydrocortisone in hormone refractory prostate cancer [abstract]. Proc ASCO 1999, 18:321a.
Picus J, Schultz M: A phase II trial of docetaxel in patients with hormone refractory cancer (HRPC): long term results [abstract]. Proc ASCO 1999, 19:314a.
Acuna LR, Langhi M, Perez J, et al.: Vinorelbine and paclitaxel as first-line chemotherapy in metastatic breast cancer. J Clin Oncol 1999, 17:74–81.
Costanza ME, Weiss RB, Henderson IC, et al.: Safety and efficacy of using a single agent or a phase II agent before instituting standard combination chemotherapy in previously untreated metastatic breast cancer patients: report of a randomized study. Cancer and Leukemia Group B 8642. J Clin Oncol 1999, 17:1397–1406.
Sparano JA, Hu P, Radha M, et al.: Phase II of doxorubicin and paclitaxel plus granulocyte colony stimulating factor in metastatic breast cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 1999, 17:3828–3834.
Iaffaioli RV, Tortoriello A, Facchini G, et al.: Phase I-II study of gemcitabine and carboplatin in stage IIIB-IV non-small-cell lung cancer. J Clin Oncol 1999, 17:921–926.
Pectasides D, Aspropotamitis A, Halikia A, et al.: Combination chemotherapy with carboplatin, docetaxel, and gemcitabine in advanced non-small cell lung cancer: a phase II study. J Clin Oncol 1999, 17:3816–3821.
Lorusso V, Carpagnano F, Frasci G, et al.: Phase I/II study of gemcitabine plus vinorelbine as first line chemotherapy of non-small-cell lung cancer. J Clin Oncol 2000, 18:405–411.
Conti JA, Kemeny NE, Saltz LB, et al.: Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 1996, 14:709–715.
Van Cutsem E, Pozzo C, Starkhammer H, et al.: A phase II study of irinotecan altered with five days bolus of 5-fluorouracil and leucovorin in first line chemotherapy of metastatic colorectal cancer. Ann Oncol 1998, 9:1199–1204.
Douillard JY, Cunningham D, Roth AD, et al.: A randomized phase III trial comparing irinotecan +5FU/Folinic acid to the same schedule of 5FU/FA in patients with metastatic colorectal cancer as first line chemotherapy [abstract]. Proc ASCO 1999, 18:233a.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelly, W.K., Slovin, S.F. Chemotherapy for androgenindependent prostate cancer: Myth or reality. Curr Oncol Rep 2, 394–401 (2000). https://doi.org/10.1007/s11912-000-0058-0
Issue Date:
DOI: https://doi.org/10.1007/s11912-000-0058-0