Abstract
Purpose of Review
Pediatric low-grade gliomas and glioneuronal tumors (pLGG) account for approximately 30% of pediatric CNS neoplasms, encompassing a heterogeneous group of tumors of primarily glial or mixed neuronal-glial histology. This article reviews the treatment of pLGG with emphasis on an individualized approach incorporating multidisciplinary input from surgery, radiation oncology, neuroradiology, neuropathology, and pediatric oncology to carefully weigh the risks and benefits of specific interventions against tumor-related morbidity. Complete surgical resection can be curative for cerebellar and hemispheric lesions, while use of radiotherapy is restricted to older patients or those refractory to medical therapy. Chemotherapy remains the preferred first-line therapy for adjuvant treatment of the majority of recurrent or progressive pLGG.
Recent Findings
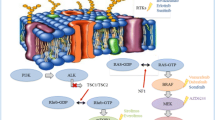
Technologic advances offer the potential to limit volume of normal brain exposed to low doses of radiation when treating pLGG with either conformal photon or proton RT. Recent neurosurgical techniques such as laser interstitial thermal therapy offer a “dual” diagnostic and therapeutic treatment modality for pLGG in specific surgically inaccessible anatomical locations. The emergence of novel molecular diagnostic tools has enabled scientific discoveries elucidating driver alterations in mitogen-activated protein kinase (MAPK) pathway components and enhanced our understanding of the natural history (oncogenic senescence). Molecular characterization strongly supplements the clinical risk stratification (age, extent of resection, histological grade) to improve diagnostic precision and accuracy, prognostication, and can lead to the identification of patients who stand to benefit from precision medicine treatment approaches.
Summary
The success of molecular targeted therapy (BRAF inhibitors and/or MEK inhibitors) in the recurrent setting has led to a gradual and yet significant paradigm shift in the treatment of pLGG. Ongoing randomized trials comparing targeted therapy to standard of care chemotherapy are anticipated to further inform the approach to upfront management of pLGG patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51. https://doi.org/10.1093/neuonc/noab106. (The updated 2021 CNS WHO classification introduced a revised classification system for adult and pediatric-type gliomas.•)
Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery. 2011;68(6):1548–54. https://doi.org/10.1227/NEU.0b013e318214a66e. (discussion 54-5).
Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–9. https://doi.org/10.1002/pbc.24958.
Packer RJ, Pfister S, Bouffet E, Avery R, Bandopadhayay P, Bornhorst M, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–61. https://doi.org/10.1093/neuonc/now209.
Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–49. https://doi.org/10.1172/JCI33656.
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12. https://doi.org/10.1038/ng.2611.
• Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569-83.e5. https://doi.org/10.1016/j.ccell.2020.03.011. (International collaborative report representing the largest cohort of clinically and molecularly annotated cohort of pLGGs that sheds light on the pLGG molecular landscape and proposes a novel risk stratification system with the potential to improve prognostication and impact treatment.•)
Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015;33(27):2986–98. https://doi.org/10.1200/JCO.2014.59.9217.
Hwang EI, Kool M, Burger PC, Capper D, Chavez L, Brabetz S, et al. Extensive molecular and clinical heterogeneity in patients with histologically diagnosed CNS-PNET treated as a single entity: a report from the Children’s Oncology Group Randomized ACNS0332 Trial. J Clin Oncol. 2018;36(34):JCO2017764720. https://doi.org/10.1200/JCO.2017.76.4720.
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–78. https://doi.org/10.1007/s00401-015-1405-4.
•• Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–22. https://doi.org/10.1016/S1470-2045(19)30277-3. (First prospective phase II study consortium-based study demonstrating promising efficacy of MEK inhibition in recurrent/refractory pediatric low-grade gliomas with and without NF1. These results led the COG to launch 2 prospective randomized phase III clinical trials for patients with newly diagnosed pediatric low-grade gliomas comparing targeted therapy versus standard of care chemotherapy in patients with and without NF1.••)
Jacob K, Quang-Khuong D-A, Jones DTW, Witt H, Lambert S, Albrecht S, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17(14):4650–60. https://doi.org/10.1158/1078-0432.Ccr-11-0127.
Bale TA, Rosenblum MK. The 2021 WHO classification of tumors of the central nervous system: an update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathology. 2022;32(4):e13060. https://doi.org/10.1111/bpa.13060.
Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–6. https://doi.org/10.1200/JCO.1993.11.5.850.
Jones DTW, Kieran MW, Bouffet E, Alexandrescu S, Bandopadhayay P, Bornhorst M, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–73. https://doi.org/10.1093/neuonc/nox141.
Giantini-Larsen AM, Pannullo S, Juthani RG. Challenges in the diagnosis and management of low-grade gliomas. World Neurosurg. 2022.
Youland RS, Khwaja SS, Schomas DA, Keating GF, Wetjen NM, Laack NN. Prognostic factors and survival patterns in pediatric low-grade gliomas over 4 decades. J Pediatr Hematol Oncol. 2013;35(3):197–205. https://doi.org/10.1097/MPH.0b013e3182678bf8.
Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397–408. https://doi.org/10.1177/0883073809342005.
Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15(8):2792–9. https://doi.org/10.1200/JCO.1997.15.8.2792.
Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–50. https://doi.org/10.1002/pbc.21563.
Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25(6):682–9. https://doi.org/10.1200/jco.2006.06.8213.
Tovar-Spinoza Z, Choi H. MRI-guided laser interstitial thermal therapy for the treatment of low-grade gliomas in children: a case-series review, description of the current technologies and perspectives. Childs Nerv Syst. 2016;32(10):1947–56. https://doi.org/10.1007/s00381-016-3193-0.
Easwaran TP, Lion A, Vortmeyer AO, Kingery K, Bc M, Raskin JS. Seizure freedom from recurrent insular low-grade glioma following laser interstitial thermal therapy. Childs Nerv Syst. 2020;36(5):1055–9. https://doi.org/10.1007/s00381-019-04493-6.
Karajannis MA, Souweidane MM, Dunkel IJ. Letter to the Editor regarding clinical debate concerning treatment of pediatric LGG by Cooney et al. Neurooncol Pract. 2020;7(5):569–70. https://doi.org/10.1093/nop/npaa019.
Revere KE, Katowitz WR, Katowitz JA, Rorke-Adams L, Fisher MJ, Liu GT. Childhood optic nerve glioma: vision loss due to biopsy. Ophthalmic Plast Reconstr Surg. 2017;33(3S Suppl 1):S107–9. https://doi.org/10.1097/IOP.0000000000000687.
Taveras JM, Mount LA, Wood EH. The value of radiation therapy in the management of glioma of the optic nerves and chiasm. Radiology. 1956;66(4):518–28. https://doi.org/10.1148/66.4.518.
Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45(1):11–5. https://doi.org/10.1016/s0167-8140(97)00102-3.
Cappelli C, Grill J, Raquin M, Pierre-Kahn A, Lellouch-Tubiana A, Terrier-Lacombe MJ, et al. Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79(4):334–8. https://doi.org/10.1136/adc.79.4.334.
Jahraus CD, Tarbell NJ. Optic pathway gliomas. Pediatr Blood Cancer. 2006;46(5):586–96. https://doi.org/10.1002/pbc.20655.
Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–34. https://doi.org/10.1093/neuonc/noq178.
Oh KS, Hung J, Robertson PL, Garton HJ, Muraszko KM, Sandler HM, et al. Outcomes of multidisciplinary management in pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2011;81(4):e481–8. https://doi.org/10.1016/j.ijrobp.2011.01.019.
Mishra KK, Puri DR, Missett BT, Lamborn KR, Prados MD, Berger MS, et al. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006;8(2):166–74. https://doi.org/10.1215/15228517-2005-011.
Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11(2):152–62. https://doi.org/10.1053/srao.2001.21421.
Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82(4):536–47. https://doi.org/10.3171/jns.1995.82.4.0536.
Raikar SS, Halloran DR, Elliot M, McHugh M, Patel S, Gauvain KM. Outcomes of pediatric low-grade gliomas treated with radiation therapy: a single-institution study. J Pediatr Hematol Oncol. 2014;36(6):e366–70. https://doi.org/10.1097/MPH.0000000000000142.
Tsang DS, Murphy ES, Merchant TE. Radiation therapy for optic pathway and hypothalamic low-grade gliomas in children. Int J Radiat Oncol Biol Phys. 2017;99(3):642–51. https://doi.org/10.1016/j.ijrobp.2017.07.023.
Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D’Angio G, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70(5):707–13. https://doi.org/10.3171/jns.1989.70.5.0707.
Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(22):3691–7. https://doi.org/10.1200/JCO.2008.21.2738.
Brauner R, Malandry F, Rappaport R, Zucker JM, Kalifa C, Pierre-Kahn A, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149(12):825–8. https://doi.org/10.1007/BF02072067.
Bowers DC, Mulne AF, Reisch JS, Elterman RD, Munoz L, Booth T, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94(4):1094–101.
Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–58. https://doi.org/10.1093/jnci/djp148.
Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, et al. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192(11):759–69. https://doi.org/10.1007/s00066-016-1005-9.
Takizawa D, Mizumoto M, Yamamoto T, Oshiro Y, Fukushima H, Fukushima T, et al. A comparative study of dose distribution of PBT, 3D-CRT and IMRT for pediatric brain tumors. Radiat Oncol. 2017;12(1):40. https://doi.org/10.1186/s13014-017-0775-2.
Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol. 2014;113(1):89–94. https://doi.org/10.1016/j.radonc.2014.08.017.
Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122(10):1483–501. https://doi.org/10.1002/cncr.29882.
Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89(5):1060–8. https://doi.org/10.1016/j.ijrobp.2014.04.053.
Indelicato DJ, Bates JE, Mailhot Vega RB, Rotondo RL, Hoppe BS, Morris CG, et al. Second tumor risk in children treated with proton therapy. Pediatr Blood Cancer. 2021;68(7):e28941. https://doi.org/10.1002/pbc.28941.
Indelicato D, Tringale K, Bradley J, Vega RM, Morris C, Casey D, et al. RONC-03. Secondary neoplasms in children with central nervous system (CNS) tumors following radiotherapy in the modern era. Neuro-Oncol. 2022;24(Suppl 1):i176.
Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, et al. Outcomes following proton therapy for pediatric low-grade glioma. Int J Radiat Oncol Biol Phys. 2019;104(1):149–56. https://doi.org/10.1016/j.ijrobp.2019.01.078.
Bitterman DS, MacDonald SM, Yock TI, Tarbell NJ, Wright KD, Chi SN, et al. Revisiting the role of radiation therapy for pediatric low-grade glioma. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(35):3335–9. https://doi.org/10.1200/JCO.19.01270.
Marcus KJ, Goumnerova L, Billett AL, Lavally B, Scott RM, Bishop K, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61(2):374–9. https://doi.org/10.1016/j.ijrobp.2004.06.012.
Cherlow JM, Shaw DWW, Margraf LR, Bowers DC, Huang J, Fouladi M, et al. Conformal radiation therapy for pediatric patients with low-grade glioma: results from the Children’s Oncology Group Phase 2 Study ACNS0221. Int J Radiat Oncol Biol Phys. 2019;103(4):861–8. https://doi.org/10.1016/j.ijrobp.2018.11.004.
Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–7. https://doi.org/10.1200/JCO.2011.36.6054.
Ryall S, Tabori U, Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. 2020;8(1):30. https://doi.org/10.1186/s40478-020-00902-z.
Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res. 2019;25(24):7303–11. https://doi.org/10.1158/1078-0432.Ccr-19-2177.
Gutmann DH, Donahoe J, Brown T, James CD, Perry A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol Appl Neurobiol. 2000;26(4):361–7.
D’Angelo F, Ceccarelli M, Tala Garofano L, Zhang J, Frattini V, et al. The molecular landscape of glioma in patients with neurofibromatosis 1. Nat Med. 2019;25(1):176–87. https://doi.org/10.1038/s41591-018-0263-8.
Evans DGR, Salvador H, Chang VY, Erez A, Voss SD, Schneider KW, et al. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res. 2017;23(12):e46–53. https://doi.org/10.1158/1078-0432.Ccr-17-0589.
Bhatia S, Chen Y, Wong FL, Hageman L, Smith K, Korf B, et al. Subsequent neoplasms after a primary tumor in individuals with neurofibromatosis type 1. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(32):3050–8. https://doi.org/10.1200/jco.19.00114.
Ullrich NJ, Robertson R, Kinnamon DD, Scott RM, Kieran MW, Turner CD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–8. https://doi.org/10.1212/01.wnl.0000257095.33125.48.
Sampson JR, Scahill SJ, Stephenson JB, Mann L, Connor JM. Genetic aspects of tuberous sclerosis in the west of Scotland. J Med Genet. 1989;26(1):28–31.
Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–7.
Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–54. https://doi.org/10.1016/j.pediatrneurol.2013.08.001.
Northrup H, Koenig MK, Pearson DA, Au KS. Tuberous sclerosis complex. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. GeneReviews(R). Seattle (WA): University of Washington, Seattle
Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19(9):716–25. https://doi.org/10.1177/08830738040190091301.
Grajkowska W, Kotulska K, Jurkiewicz E, Matyja E. Brain lesions in tuberous sclerosis complex. Review Folia neuropathologica. 2010;48(3):139–49.
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (New York NY). 1997;277(5327):805–8.
Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med Off J Am Coll Med Genet. 2007;9(2):88–100. https://doi.org/10.1097/GIM.0b013e31803068c7.
Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–34. https://doi.org/10.1038/nrc1974.
Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99(21):13571–6. https://doi.org/10.1073/pnas.202476899.
Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–8. https://doi.org/10.1177/088307389801301206.
Krueger DA. Management of CNS-related disease manifestations in patients with tuberous sclerosis complex. Curr Treat Options Neurol. 2013;15(5):618–33. https://doi.org/10.1007/s11940-013-0249-2.
Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology. 2004;63(8):1457–61.
Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–8. https://doi.org/10.1002/ana.20784.
Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–11. https://doi.org/10.1056/NEJMoa1001671.
Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–32. https://doi.org/10.1016/S0140-6736(12)61134-9.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14(8):843–8. https://doi.org/10.1038/nm1788.
Wiegand G, May TW, Ostertag P, Boor R, Stephani U, Franz DN. Everolimus in tuberous sclerosis patients with intractable epilepsy: a treatment option? Eur J Paediatr Neurol. 2013;17(6):631–8. https://doi.org/10.1016/j.ejpn.2013.06.002.
Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–80. https://doi.org/10.1212/WNL.0b013e3182815428.
Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–7. https://doi.org/10.1158/0008-5472.CAN-08-2097.
Ryall S, Arnoldo A, Krishnatry R, Mistry M, Khor K, Sheth J, et al. Multiplex detection of pediatric low-grade glioma signature fusion transcripts and duplications using the NanoString nCounter System. J Neuropathol Exp Neurol. 2017;76(7):562–70. https://doi.org/10.1093/jnen/nlx042.
Becker AP, Scapulatempo-Neto C, Carloni AC, Paulino A, Sheren J, Aisner DL, et al. KIAA1549: BRAF gene fusion and FGFR1 hotspot mutations are prognostic factors in pilocytic astrocytomas. J Neuropathol Exp Neurol. 2015;74(7):743–54. https://doi.org/10.1097/nen.0000000000000213.
Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119(5):641–9. https://doi.org/10.1007/s00401-009-0634-9.
Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol. 2017;35(25):2934–41. https://doi.org/10.1200/jco.2016.71.8726.
Cin H, Meyer C, Herr R, Janzarik WG, Lambert S, Jones DTW, et al. Oncogenic FAM131B–BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–74. https://doi.org/10.1007/s00401-011-0817-z.
Helgager J, Lidov HG, Mahadevan NR, Kieran MW, Ligon KL, Alexandrescu S. A novel GIT2-BRAF fusion in pilocytic astrocytoma. Diagn Pathol. 2017;12(1):82. https://doi.org/10.1186/s13000-017-0669-5.
Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–32. https://doi.org/10.1038/ng.2682.
Fangusaro J, Onar-Thomas A, Poussaint TY, Lensing S, Wu S, Ligon AH, et al. LGG-06. Selumetinib in pediatric patients with non-neurofibromatosis type 1-associated, non-optic pathway (OPG) and non-pilocytic recurrent/progressive low-grade glioma harboring BRAFV600E mutation or BRAF-KIAA1549 fusion: a multicenter prospective Pediatric Brain Tumor Consortium (PBTC) Phase 2 trial. Neuro-Oncol. 2022;24(Supplement_1):i88-i. https://doi.org/10.1093/neuonc/noac079.322.
Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, et al. LGG-02. A phase II Prospective Trial Of Selumetinib In Children With Recurrent/Progressive pediatric low-grade glioma (pLGG) with a focus upon optic pathway/hypothalamic tumors and visual acuity outcomes: a pediatric brain tumor consortium (PBTC) study, PBTC-029B. Neuro-Oncol. 2019;21(Supplement_2):ii98–9. https://doi.org/10.1093/neuonc/noz036.145.
Robison N, Pauly J, Malvar J, Gardner S, Allen J, Margol A, et al. LTBK-04. LATE BREAKING ABSTRACT: MEK162 (binimetinib) in children with progressive or recurrent low-grade glioma: a multi-institutional phase II and target validation study. Neuro-Oncol. 2022;24(Supplement_1):i191–2. https://doi.org/10.1093/neuonc/noac079.716.
Perreault S, Sadat Kiaei D, Dehaes M, Larouche V, Tabori U, Hawkin C, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway. J Clin Oncol. 2022;40(16_suppl):2042. https://doi.org/10.1200/JCO.2022.40.16_suppl.2042.
Trippett T, Toledano H, Campbell Hewson Q, Verschuur A, Langevin AM, Aerts I, et al. Cobimetinib in pediatric and young adult patients with relapsed or refractory solid tumors (iMATRIX-cobi): a multicenter, phase I/II study. Target Oncol. 2022;17(3):283–93. https://doi.org/10.1007/s11523-022-00888-9.
Fangusaro J, Onar-Thomas A, Wu S, Poussaint TY, Packer R, Kilburn L, et al. LGG-04. A phase II re-treatment study of selumetinib for recurrent or progressive pediatric low-grade glioma (pLGG): a pediatric brain tumor consortium (PBTC) study. Neuro-Oncol. 2020;22(Supplement_3):iii367-iii. https://doi.org/10.1093/neuonc/noaa222.389.
Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10(3):385–94. https://doi.org/10.1158/1535-7163.Mct-10-0799.
Yao Z, Torres NM, Tao A, Gao Y, Luo L, Li Q, et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell. 2015;28(3):370–83. https://doi.org/10.1016/j.ccell.2015.08.001.
Gierke M, Sperveslage J, Schwab D, Beschorner R, Ebinger M, Schuhmann MU, et al. Analysis of IDH1-R132 mutation, BRAF V600 mutation and KIAA1549–BRAF fusion transcript status in central nervous system tumors supports pediatric tumor classification. J Cancer Res Clin Oncol. 2016;142(1):89–100. https://doi.org/10.1007/s00432-015-2006-2.
Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. https://doi.org/10.1007/s00401-011-0802-6.
Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley M-YC, Yu M, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70(2):512–9. https://doi.org/10.1158/0008-5472.CAN-09-1851.
Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol. 2017;35(25):2934–41. https://doi.org/10.1200/JCO.2016.71.8726.
Dahiya S, Haydon DH, Alvarado D, Gurnett CA, Gutmann DH, Leonard JR. BRAFV600E mutation is a negative prognosticator in pediatric ganglioglioma. Acta Neuropathol. 2013;125(6):901–10. https://doi.org/10.1007/s00401-013-1120-y.
Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol. 2015;33(9):1015–22. https://doi.org/10.1200/jco.2014.58.3922.
Tanaka S, Nakada M, Nobusawa S, Suzuki SO, Sabit H, Miyashita K, et al. Epithelioid glioblastoma arising from pleomorphic xanthoastrocytoma with the BRAF V600E mutation. Brain Tumor Pathol. 2014;31(3):172–6. https://doi.org/10.1007/s10014-014-0192-2.
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–78. https://doi.org/10.1007/s00401-015-1405-4.
Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23(1):53–64. https://doi.org/10.1016/S1470-2045(21)00578-7.
• Salama AKS, Li S, Macrae ER, Park JI, Mitchell EP, Zwiebel JA, et al. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH trial subprotocol H. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(33):3895–904. https://doi.org/10.1200/jco.20.00762. (Subprotocol H (EAY131-H) of the NCI-MATCH platform trial was a single-arm phase II histology agnostic trial investigating the combination of BRAF inhibitor dabrafenib and the MEK1/2 inhibitor trametinib in a biomarker-selected cohort of patients with recurrent/refractory solid tumors harboring a BRAFV600 mutation. Dabrafenib and trametinib therapy resulted in responses in 38% of patients and showed a high rate of disease control across a variety of disease histologies eventually culminating in the recent FDA approval of dabrafenib and trametinib for patients with BRAFV600E-mutant solid tumors.•)
•• Bouffet E, Geoerger B, Moertel C, Whitlock JA, Aerts I, Hargrave D, et al. Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J Clin Oncol. 2023;41(3):664–74. https://doi.org/10.1200/JCO.22.01000. (This randomized phase 2 trial tested dabrafenib with trametinib versus standard-of-care chemotherapy (carboplatin/vincristine) and demonstrated improved overall response rate (ORR) and prolonged progression-free survival (PFS) with targeted therapy compared with standard chemotherapy. Dabrafenib with trametinib represents a new standard of care for pediatric patients with newly diagnosed BRAFV600-mutant low-grade glioma.•)
Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–16. https://doi.org/10.1093/neuonc/nou059.
Sievert AJ, Lang SS, Boucher KL, Madsen PJ, Slaunwhite E, Choudhari N, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110(15):5957–62. https://doi.org/10.1073/pnas.1219232110.
Clarke M, Mackay A, Ismer B, Pickles JC, Tatevossian RG, Newman S, et al. Infant high-grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov. 2020;10(7):942–63. https://doi.org/10.1158/2159-8290.CD-19-1030.
Gessi M, Moneim YA, Hammes J, Goschzik T, Scholz M, Denkhaus D, et al. FGFR1 mutations in Rosette-forming glioneuronal tumors of the fourth ventricle. J Neuropathol Exp Neurol. 2014;73(6):580–4. https://doi.org/10.1097/nen.0000000000000080.
Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK, et al. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019;138(3):497–504. https://doi.org/10.1007/s00401-019-02038-4.
Lasorella A, Sanson M, Iavarone A. FGFR-TACC gene fusions in human glioma. Neuro Oncol. 2017;19(4):475–83. https://doi.org/10.1093/neuonc/now240.
Huse JT, Snuderl M, Jones DT, Brathwaite CD, Altman N, Lavi E, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–29. https://doi.org/10.1007/s00401-016-1639-9.
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–45. https://doi.org/10.1007/s00401-016-1539-z.
Farouk Sait S, Gilheeney SW, Bale TA, Haque S, Dinkin MJ, Vitolano S, et al. Debio1347, an oral FGFR inhibitor: results from a single-center study in pediatric patients with recurrent or refractory FGFR-altered gliomas. JCO Precis Oncol. 2021;5. https://doi.org/10.1200/po.20.00444.
Bandopadhayay P, Ramkissoon LA, Jain P, Bergthold G, Wala J, Zeid R, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–82. https://doi.org/10.1038/ng.3500.
Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110(20):8188–93. https://doi.org/10.1073/pnas.1300252110.
Cicirò Y, Sala A. MYB oncoproteins: emerging players and potential therapeutic targets in human cancer. Oncogenesis. 2021;10(2):19. https://doi.org/10.1038/s41389-021-00309-y.
Chan E, Bollen AW, Sirohi D, Van Ziffle J, Grenert JP, Kline CN, et al. Angiocentric glioma with MYB-QKI fusion located in the brainstem, rather than cerebral cortex. Acta Neuropathol. 2017;134(4):671–3. https://doi.org/10.1007/s00401-017-1759-x.
Chiang J, Harreld JH, Tinkle CL, Moreira DC, Li X, Acharya S, et al. A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol. 2019;138(6):1091–2. https://doi.org/10.1007/s00401-019-02081-1.
D’Aronco L, Rouleau C, Gayden T, Crevier L, Décarie J-C, Perreault S, et al. Brainstem angiocentric gliomas with MYB–QKI rearrangements. Acta Neuropathol. 2017;134(4):667–9. https://doi.org/10.1007/s00401-017-1763-1.
Li KW, Roonprapunt C, Lawson HC, Abbott IR, Wisoff J, Epstein F, et al. Endoscopic third ventriculostomy for hydrocephalus associated with tectal gliomas. Neurosurg Focus. 2005;18(6a):E2.
Epstein F, Constantini S. Practical decisions in the treatment of pediatric brain stem tumors. Pediatr Neurosurg. 1996;24(1):24–34. https://doi.org/10.1159/000121011.
Liu APY, Harreld JH, Jacola LM, Gero M, Acharya S, Ghazwani Y, et al. Tectal glioma as a distinct diagnostic entity: a comprehensive clinical, imaging, histologic and molecular analysis. Acta Neuropathol Commun. 2018;6(1):101. https://doi.org/10.1186/s40478-018-0602-5.
Lapras C, Bognar L, Turjman F, Villanyi E, Mottolese C, Fischer C, et al. Tectal plate gliomas. Part I: microsurgery of the tectal plate gliomas. Acta Neurochir (Wien). 1994;126(2–4):76–83. https://doi.org/10.1007/bf01476414.
May PL, Blaser SI, Hoffman HJ, Humphreys RP, Harwood-Nash DC. Benign intrinsic tectal “tumors” in children. J Neurosurg. 1991;74(6):867–71. https://doi.org/10.3171/jns.1991.74.6.0867.
Chiang J, Li X, Liu APY, Qaddoumi I, Acharya S, Ellison DW. Tectal glioma harbors high rates of KRAS G12R and concomitant KRAS and BRAF alterations. Acta Neuropathol. 2020;139(3):601–2. https://doi.org/10.1007/s00401-019-02112-x.
Dağlioğlu E, Cataltepe O, Akalan N. Tectal gliomas in children: the implications for natural history and management strategy. Pediatr Neurosurg. 2003;38(5):223–31. https://doi.org/10.1159/000069823.
Disabato JA, Handler MH, Strain JD, Fleitz JM, Foreman NK. Successful use of intracavitary bleomycin for low-grade astrocytoma tumor cyst. Pediatr Neurosurg. 1999;31(5):246–50. https://doi.org/10.1159/000028871.
Giovanini MA, Mickle JP. Long-term access to cystic brain stem lesions using the Ommaya reservoir: technical case report. Neurosurg. 1996;39(2):404–7. https://doi.org/10.1097/00006123-199608000-00039. (discussion 7-8).
Perwein T, Benesch M, Kandels D, Pietsch T, Schmidt R, Quehenberger F, et al. High frequency of disease progression in pediatric spinal cord low-grade glioma (LGG): management strategies and results from the German LGG study group. Neuro Oncol. 2021;23(7):1148–62. https://doi.org/10.1093/neuonc/noaa296.
Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–54. https://doi.org/10.3171/jns.1997.86.5.0747.
Mahoney DH Jr, Cohen ME, Friedman HS, Kepner JL, Gemer L, Langston JW, et al. Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2000;2(4):213–20. https://doi.org/10.1093/neuonc/2.4.213.
Laithier V, Grill J, Le Deley MC, Ruchoux MM, Couanet D, Doz F, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy–results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(24):4572–8. https://doi.org/10.1200/jco.2003.03.043.
Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–84. https://doi.org/10.1093/neuonc/nos202.
Chintagumpala M, Eckel SP, Krailo M, Morris M, Adesina A, Packer R, et al. A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a Children’s Oncology Group study†. Neuro Oncol. 2015;17(8):1132–8. https://doi.org/10.1093/neuonc/nov057.
Gnekow AK, Walker DA, Kandels D, Picton S, Giorgio P, Grill J, et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - a final report. Eur J Cancer. 2017;81:206–25. https://doi.org/10.1016/j.ejca.2017.04.019.
Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(29):3537–43. https://doi.org/10.1200/jco.2016.68.1585.
Funding
This work has been supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. Karajannis reports grants from Y-mAbs Therapeutics (research support) and personal fees from Bayer, AstraZeneca, QED Therapeutics, CereXis, Recursion, Alexion, Cardinal Health, and Medscape (medical advisory board and/or consultant). Dr. Tringale reports grants from Hopper Belmont Foundation and RSNA Resident Grant, as well as honoraria from GT Medical Technologies. Drs. Farouk Sait and Souweidane report no relationships, conditions, or circumstances that present a potential conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sait, S.F., Giantini-Larsen, A.M., Tringale, K.R. et al. Treatment of Pediatric Low-Grade Gliomas. Curr Neurol Neurosci Rep 23, 185–199 (2023). https://doi.org/10.1007/s11910-023-01257-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01257-3
Keywords
Profiles
- Matthias A. Karajannis View author profile