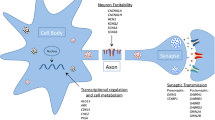
Abstract
Genetic factors are likely to play a major role in many epileptic conditions, spanning from classical idiopathic (genetic) generalized epilepsies to epileptic encephalopathies and focal epilepsies. In this review we describe the genetic advances in progressive myoclonus epilepsies, which are strictly monogenic disorders, genetic generalized epilepsies, mostly exhibiting complex genetic inheritance, and SCN1A-related phenotypes, namely genetic generalized epilepsy with febrile seizure plus and Dravet syndrome. Particular attention is devoted to a form of familial focal epilepsies, autosomal-dominant lateral temporal epilepsy, which is a model of non-ion genetic epilepsies. This condition is associated with mutations of the LGI1 gene, whose protein is secreted from the neurons and exerts its action on a number of targets, influencing cortical development and neuronal maturation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marseille Consensus Group. Classification of progressive myoclonus epilepsies and related disorders. Ann Neurol. 1990;28:113–6.
Michelucci R, Serratosa JM, Genton P, Tassinari CA. Seizures, myoclonus and cerebellar dysfunction in progressive myoclonus epilepsies. In: Guerrini R, Aicardi J, Andermann F, Hallet M, editors. In epilepsy and movement disorders. Cambridge: Cambridge University Press; 2002. p. 227–49.
Magaudda A, Ferlazzo E, Nguyen VH, Genton P. Unverricht-Lundborg disease, a condition with self limited progression: long-term follow-up of 20 patients. Epilepsia. 2006;47:860–6.
Pennacchio LA, Lehesjoki AE, Stone NE, et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science. 1996;271:1731–4.
Lalioti MD, Scott HS, Buresi C, et al. Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature. 1997;386:847–51.
Joensuu T, Lehesjoki AE, Kopra O. Molecular background of EPM1—Unverricht-Lundborg disease. Epilepsia. 2008;49:557–63.
• Koskenkorva P, Hypponene J, Aikia M, et al. Severer phenotype in Unverricht-Lundborg disease (EPM1) patients compound heterozygotes for the dodecamer repeat expansion and the c.202C>T mutation in the CSTB gene. Neurodegenerative Dis. 2011;8:515–22. The authors describe five Finnish compound heterozygotes for the dodecamer repeat expansion and the c.202C>T mutations, who showed a lower age of onset, a more severe form of myoclonus, drug-resistant seizures, and lower cognitive performances than homozygous patients.
Berkovic SF, Mazarib A, Walid S, et al. A new clinical and molecular form of Unverricht-Lundborg disease localized by homozygosity mapping. Brain. 2005;128:652–8.
• Bassuk AG, Wallace RH, Buhr A, et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet. 2008;83:572–81. In three families with PME and ataxia unrelated to known PME loci, a mutation in PRICKLE1 gene was detected. At variance with ULD, the patients had an early onset of ataxia (around age 4 years) and seizures (5–10 years). PRICKLE1 was the first molecule in the noncanonical WNT signaling pathway to be directly implicated in human epilepsy.
•• Dibbens LM, Michelucci R, Gambardella A, et al. SCARB2 mutations in progressive myoclonyus epilepsy (PME) without renal failure. Ann Neurol. 2009;66:532–6. In a multicenter retrospective study aimed to discover new loci/genes in unsolved cases of PME, the authors found mutations in SCARB2, a gene previously found to be associated with AMRF, in five Italian patients. Four of these patients were dead at the time of the study, but did not show any sign of renal impairment even after many years of evolution.
• Rubboli G, Franceschetti S, Berkovic SF, et al. Clinical and neurophysiological features of progressive myoclonus epilepsy without renal failure caused by SCARB2 mutations. Epilepsia. 2011;52:2356–63. Compared with ULD, these five Italian SCARB2-mutated patients had a more variable age of onset (spanning from adolescence to early adulthood), a more severe course with resistant seizures, loss of motor autonomy, and death occurring after a period of 10 to 15 years from the onset. Relevant neurophysiological findings included pronounced photosensitivity and rhythmic myoclonic jerks at 12 to 20 Hz clinically resembling a postural tremor.
Berkovic SF, Dibbens LM, Oshlack A, et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:673–84.
Minassian BA, Lee JR, Herbrick JA, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Gen. 1998;20:171–4.
Serratosa JM, Gomez-Garre P, Galabido ME, et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of Lafora type (EPM2). Hum Mol Genet. 1999;8:345–52.
Chan EM, Young EJ, Ianzano L, et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet. 2003;35:125–7.
Ramachandran N, Girard JM, Turnbull J, Minassian BA. The autosomal recessively inherited progressive myoclonus epilepsies and their genes. Epilepsia. 2009;30(Suppl5):29–36.
•• Turnbull J, DePaoli-Roach AA, Zhao X, et al. PTG depletion removes Lafora bodies and rescues the fatal epilepsy of Lafora disease. PLoS Genet. 2011;7:e1002037. The authors generated double knockout mice deficient in laforin and PTG, a protein involved in activating glycogen synthase. These double knockout mice had almost no polyglucosans, no neurodegeneration, and no seizures and resulted in a cure for this disease.
• Guerrero R, Vernia S, Sanz R, et al. A PTG variant contributes to a milder phenotype in Lafora disease. PLoS Genet. 2011;6:e21294. One patient with an unusually mild phenotype of LD and EPM2B mutation also had mutation of the PPP1R3C gene encoding PTG. This mutation (c.746A>G, N249S) resulted in a decreased capacity to induce glycogen synthesis and reduced interaction with glycogen phosphorylase and laforin, supporting a key role of PTG as a potential target for pharmacogenetic and therapeutic approaches.
Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33:42–63.
Berkovic SF, Carpenter S, Andermann F, et al. Kufs’ disease: a critical reappraisal. Brain. 1988;111:27–62.
Arsov T, Smith KR, Damiano J, et al. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88:566–73.
Noskova L, Stranecky V, Hartmannova H, et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet. 2011;89:241–52.
Zara F, Bianchi A, Avanzini G, et al. Mapping of genes predisposing to idiopathic generalized epilepsy. Hum Mol Genet. 1995;4:1201–7.
Sander T, Schulz H, Saar K, et al. Genome search for susceptibility loci of common idiopathic generalised epilepsies. Hum Mol Genet. 2000;9:1465–72.
Durner M, Keddache MA, Tomasini L, et al. Genome scan of idiopathic generalized epilepsy: evidence for major susceptibility gene and modifying genes influencing the seizure type. Ann Neurol. 2001;49:328–35.
Hampelmann A, Taylor KP, Heils A, et al. Exploration of the genetic architecture of idiopathic generalized epilepsies. Epilepsia. 2006;47:1682–90.
Chioza BA, Aicardi J, Aschauer H, et al. Genome wide high density SNP-based linkage analysis of childhood absence epilepsy identifies a susceptibility locus on chromosome 3p23-p14. Epilepsy Res. 2009;87:247–55.
Greenberg DA, Subaran R. Blinders, phenotype, and fashionable genetic analysis: a critical examination of the current state of epilepsy genetic studies. Epilepsia. 2011;52:1–9.
•• Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–48. These authors performed systematic exomic resequencing of 237 ion channel genes in a sample of sporadic cases with idiopathic epilepsy and in matched controls to identify rare variants conferring risk for epilepsy. Their findings delineate a remarkably complex genetic architecture of rare and common variants in each affected individual, in which the risk conferred even by deleterious ion channel mutations depends on the other variants with which they are combined. Variant discovery by large-scale sequencing seems to be only the fist step toward elucidation of personal disease risk.
• EPICURE Consortium, Leu C, de Kovel CGF, et al. Genome-wide linkage meta-analysis identifies susceptibility loci at 2q34 and 13q31.3 for genetic generalized epilepsies. Epilepsia. 2012;53:308–18. In this meta-analysis of three genome-wide studies carried out in 379 multiplex families of European ancestry, two family subgroups (with predominantly genetic absence epilepsy and juvenile myoclonic epilepsy) were stratified. While a locus at 5q34 conferred risk to a broad spectrum of familial GGEs, susceptibility loci at 2q34 and 13q31.3 preferentially predisposed to myoclonic seizures or absence seizures.
Helbig I, Mefford HC, Sharp AJ, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–2.
Dibbens LM, Mullen S, Helbig I, et al. Familial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: precedent for disorders with complex inheritance. Hum Mol Genet. 2009;18:3626–31.
De Vivo DC, Trifiletti RR, Jacobson RI, et al. Defective glucose transport across the blood–brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325:703–9.
Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57:111–8.
Suls A, Mullen SA, Weber YG, et al. Early-onset absence epilepsy caused by mutations in the glucose transporter GLUT1. Ann Neurol. 2009;66:415–9.
Mullen SA, Suls A, De JP, et al. Absence epilepsies with widely variable onset as a key feature of familial GLUT1 deficiency. Neurology. 2010;75:432–40.
• Striano P, Weber YG, Toliat MR, et al. GLUT1 mutations are a rare cause of idiopathic generalized epilepsy. Neurology. 2012;78:557–62. The authors screened the SLC2A1 gene, encoding GLUT1, for mutations in a group of 95 European patients with familial idiopathic generalized epilepsy and found one novel mutation in one family. Nine family members were affected mainly by absence epilepsies with a variable age of onset from early childhood to adulthood.
Mullen SA, Marini C, Suls A, et al. Glucose transporter 1 deficiency as a treatable cause of myoclonic astatic epilepsy. Arch Neurol. 2011, 1152–55.
Scheffer I, Berkovic SF. Generalized (genetic) epilepsy with febrile seizure plus. In: Engel JJ, Pedley T, editors. In epilepsy. A comprehensive textbook. Philadelphia: Lippincott, Williams & Wilkins; 2008. p. 2553–8.
Singh R, Andermann E, Whitehouse WPA, et al. Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+? Epilepsia. 2001;42:837–44.
Scheffer IE, Harkin LA, Grinton BE, et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–9.
Thomas RH, Johnston JA, Hammond CL, et al. Genetic epilepsy with febrile seizure plus: definite and borderline phenotypes. J Neurol Neurosurg Psychiatry. 2012;83:336–8.
Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+ channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–70.
Escayg A, MasDonald BT, Meeislerr MH, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–5.
Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet. 2001;28:46–8.
Claes L, Del-Favero J, Ceulemans B, et al. De novo mutations in the sodium channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32.
Depienne C, Trouillard O, Saint-martin C, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46:183–91.
Fujiwara T, Sugawara T, Mazaki-Miyazaki E, et al. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain. 2003;126:531–46.
• Catarino C, Liu JYW, Liagkouras I, et al. Dravet syndrome as epileptic encephalopathy: evidence from long-term course and neuropathology. Brain. 2011;134:2982–3010. The authors report 22 adult patients with DS, including three postmortem cases. In 60 % of cases SCN1A, mostly novel, mutations were identified. Features in adulthood included multiple seizure types, fever sensitivity, cognitive and motor deterioration, and dysphagia. Treatment changes improved seizure control and cognitive performance.
Harkin L, McMahon JM, Iona X, et al. The spectrum of SCN1A-related infantile epileptic encepahlopathies. Brain. 2007;130:843–52.
Mulley JC, Nelson P, Guerrero S, et al. A new molecular mechanism for severe muyoclonic epilepsy of infancy: exonic deletions in SCN1A. Neurology. 2006;67:1094–5.
Wang JW, Kurahashi H, Ishii A, et al. Microchromosomal deletions involving SCN1A and adjacent genes in severe myoclonic epilepsy in infancy. Epilepsia. 2008;49:1528–34.
Singh NA, Pappas C, Dahle EJ, et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PloS Genet. 2009;5:e1000649.
Marini C, Mei D, Cross H, et al. Mosaic SCN1A mutation in familial severe myoclonic epilepsy in infancy. Epilepsia. 2006;47:1737–40.
Depienne C, Trouillard O, Gourfinkel-An I, et al. Mechanisms for variable expressivity of inherited SCN1A mutations causing Dravet syndrome. J Med Genet. 2010;47:404–10.
De Jonghe P. Molecular genetics of Dravet syndrome. Dev Med Child Neurol. 2011;53(suppl2):7–10.
Shi YW, Yu MJ, Long YS, et al. Mosaic SCN1A mutations in familial partial epilepsy with antecedent febrile seizures. Gene Brain Behav. 2012;11:170–6.
Carranza Rojo D, Hamiwka L, McMahon JM, et al. De novo SCN1A mutations in migrating partial seizures in infancy. Neurology. 2011;77:380–3.
Zuberi SM, Brunklaus A, Birch R, et al. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology. 2011;76:594–600.
Depienne C, Bouteiller D, Keren B, et al. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 2009;5:e1000381.
Dibbens LM, Tarpey PS, Hynes K, et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat Genet. 2008;40:776–81.
Depienne C, Trouillard O, Bouteiller D, et al. Mutations and deletions in PCDH19 account for various familial or isolated epilepsies in females. Hum Mutat. 2011;32:e1959–75.
• Marini C, Mei D, Parmeggiani L, et al. Protocadherin 19 mutations in girls with infantile-onset epilepsy. Neurology. 2010;75:646–53. The authors screened for PCDH19 mutations 117 female patients with febrile seizures and a wide spectrum of epilepsy phenotypes including focal and generalized forms with either sporadic or familial distribution. They found mutation in 13 (11 %) probands whose phenotype included epileptic encephalopathy with DS-like features and focal epilepsy of variable severity, usually with a stormy onset.
Specchio N, Marini C, Terracciano A, et al. Spectrum of phenotypes in female patients with epilepsy due to protocadherin 19 mutations. Epilepsia. 2011;52:1251–7.
Roll P, Rudolf G, Pereira S, et al. SRPX mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–207.
Kinton L, Johnson MR, Smith SJ, et al. Partial epilepsy with pericentral spikes: a new familial epilepsy syndrome with evidence for linkage to chromosome 4p15. Ann Neurol. 2002;51:740–9.
Deprez L, Peeters K, Van Paesschen W, et al. Familial occipitotemporal lobe epilepsy and migraine with visual aura—linkage to chromosome 9q. Neurology. 2007;68:1995–2002.
Scheffer IE, Bhatia KP, Lopes-Cendes I, et al. Autosomal dominant nocturnal frontal lobe epilepsy. A distintive clinical disorder. Brain. 1995;118:61–73.
Seinlein OK, Magnusson A, Stoodt J, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–3.
De Fusco M, Becchetti A, Patrignani A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26:275–6.
Phillips HA, Favre I, Kirkpatrick M, et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2011;68:225–31.
Crompton DE, Scheffer IE, Taylor I, et al. Familial mesial temporal lobe epilepsy: a benign epilepsy syndrome showing complex inheritance. Brain. 2010;133:3221–31.
Hedera P, Picard F, Herman A, et al. Familial mesial temporal lobe epilepsy maps to chromosome 4q13.2-q21.3. Neurology. 2007;68:786–92.
Azmanov DN, Zhelyazkova S, Radionova M, et al. Focal epilepsy of probable temporal lobe origin in a gypsy family showing linkage to a novel locus on 7p21.3. Epilepsy Res. 2011;96:101–8.
Kobayashi E, Lopes-Cendes I, Guerreiro CA, et al. Seizure outcome and hippocampal atrophy in familial mesial temporal lobe epilepsy. Neurology. 2001;56:166–72.
Baulac S, Picard F, Herman A, et al. Evidence for digenic inheritance in a family with both febrile convulsions and temporal lobe epilepsy implicating chromosomes 18qter and 1q25-q31. Ann Neurol. 2001;49:786–92.
Claes L, Audenaert D, Deprez L, et al. Novel locus on chromosome 12q22-q23.3 responsible for familial temporal lobe epilepsy associated with febrile seizures. J Med Genet. 2004;41:710–4.
Ottman R, Risch N, Hauser WA, et al. Localization of a gene for partial epilepsy to chromosome 10q. Nat Genet. 1995;10:56–60.
Poza JJ, Saenz A, Martinez-Gil A, et al. Autosomal dominant lateral temporal lobe epilepsy: clinical and genetic study of a large basque pedigree linked to chromosome 10q. Ann Neurol. 1999;45:182–8.
Brodtkorb E, Gu W, Nakken KO, et al. Familial temporal lobe epilepsy with aphasic seizures and linkage to chromosome 10q22-q24. Epilepsia. 2002;43:228–35.
Michelucci R, Passarelli D, Pitzalis S, et al. Autosomal dominant partial epilepsy with auditory features: description of a new family. Epilepsia. 2000;41:967–70.
Michelucci R, Poza JJ, Sofia V, et al. Autosomal dominant lateral temporal epilepsy: clinical spectrum, new Epitempin mutations, and genetic heterogeneity in seven European families. Epilepsia. 2003;44:1289–97.
Ottman R, Winawer MR, Kalachikov S, et al. LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology. 2004;62:1120–6.
Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–28.
Kalachikov S, Evgrafov O, Ross B, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–41.
Nobile C, Michelucci R, Andreazza S, et al. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 2009;30:530–6.
Bisulli F, Tinuper P, Scudellaro E, et al. A de novo LGI1 mutation in sporadic partial epilepsy with auditory features. Ann Neurol. 2004;56:455–6.
Michelucci R, Mecarelli O, Bovo G, et al. A de novo LGI1 mutation causing idiopathic partial epilepsy with telephone-induced seizures. Neurology. 2007;68:2150–1.
Di Bonaventura C, Operto FF, Busolin G, et al. Low penetrance and effect on protein secretion of LGI1 mutations causing autosomal dominant lateral temporal epilepsy. Epilepsia. 2011;52:1258–64.
Michelucci R, Pasini E, Nobile C. Lateral temporal lobe epilepsies: clinical and genetic features. Epilepsia. 2009;50 Suppl 5:52–4.
Kobe B, Kajava AV. The leucine rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–32.
Staub E, Perez-Tur J, Siebert R, et al. The novel EPTP repeat defines a superfamily of proteins with implications in epileptic disorders. Trends Biochem Sci. 2002;27:441–4.
Senechal KR, Thaller C, Noebels JL. ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum Mol Genet. 2005;14:1613–20.
Sirerol-Piquer MS, Ayerdi-Izquierdo A, Morante-Redolat JM, et al. The epilepsy gene LGI1 encodes a secreted glycoprotein that binds to the cell surface. Hum Mol Genet. 2006;15:3436–45.
Chabrol E, Popescu C, Gourfinkel-An I, et al. Two novel epilepsy-linked mutations leading to a loss of function of LGI1. Arch Neurol. 2007;64:217–22.
Striano P, de Falco A, Diani E, et al. A novel loss-of-function LGI1 mutation linked to autosomal dominant lateral temporal epilepsy. Arch Neurol. 2008;65:939–42.
•• Striano P, Busolin G, Santulli L, et al. Familial temporal lobe epilepsy with psychic auras associated with a novel LGI1 mutation. Neurology. 2011;76:1173–6. The finding of an LGI1 mutation associated with symptoms suggestive of mesial temporal origin expands the range of auras linked to this gene. Interestingly, this is the first mutation that does not inhibit secretion of the mutant protein, but it is not clear whether this functional effect is related to the atypical familial phenotype.
Leonardi E, Andreazza S, Vanin S, et al. A computational model of the LGI1 protein suggests a commom binding site for ADAM proteins. PLoS One. 2011;6:e18142.
Striano P, Gambardella A, Coppola A, et al. Familial mesial temporal lobe epilepsy (FMTLE): a clinical and genetic study of 15 italian families. J Neurol. 2008;255:16–23.
Berkovic SF, Izzillo P, McMahon JM, et al. LGI1 mutations in temporal lobe epilepsies. Neurology. 2004;62:1115–9.
Fukata Y, Adesnik H, Iwanaga T, et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313:1792–5.
Schulte U, Thumfart JO, Klocker N, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49:697–706.
Owuor K, Harel NY, Englot DJ, et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 2009;42:448–57.
Fukata Y, Lovero KL, Iwanaga T, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci. 2010;107:3799–804.
Zhou YD, Lee S, Jin Z, et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–14.
•• Zhou YD, Zhang D, Ozkaynak E, et al. Epilepsy gene LGI1 regulates postnatal developmental remodeling of retinogeniculate synapses. J Neurosci. 2012;32:903–10. This paper shows that postnatal synapse refinement is inhibited in developing the visual system of mice with truncated mutant LGI1 or heterozygous LGI1 knockout. These findings suggest that a similarly impaired synapse refinement in the developing auditory system could provide a potential explanation for aberrant sensory processing in LGI1-mutated epileptic patients.
Michelucci R, Gardella E, de Haan GJ, et al. Telephone-induced seizures: a new type of reflex epilepsy. Epilepsia. 2004;45:280–3.
Berkovic SF, Serratosa JM, Phillips HA, et al. Familial partial epilepsy with variable foci: clinical features and linkage to chromosome 22q12. Epilepsia. 2004;45:1054–60.
Ottman R, Hirose S, Jain S, et al. Genetic testing in the epilepsies—report of the ILAE genetics commission. Epilepsia. 2010;51:655–70.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michelucci, R., Pasini, E., Riguzzi, P. et al. Genetics of Epilepsy and Relevance to Current Practice. Curr Neurol Neurosci Rep 12, 445–455 (2012). https://doi.org/10.1007/s11910-012-0281-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0281-8