Abstract
Purpose of Review
Japanese encephalitis (JE), a clinical indication of JE virus–induced brain inflammation, is the most prevalent cause of viral encephalitis in the world. This review gives a comprehensive update on the epidemiology, clinical features, therapeutic trials and approaches for preventing the spread of JE. It also outlines the different JE vaccines used in various countries and recommendations for administration of JE vaccines.
Recent Findings
According to the WHO, annual incidence of JE is estimated to be approximately 68,000 cases worldwide. It is widespread across Asia–Pacific, with a potential for worldwide transmission. In endemic locations, JE is believed to affect children below 6 years of age, but in newly affected areas, both adults and children are at risk due to a lack of protective antibodies. Various vaccines have been developed for the prevention of JE and are being administered in endemic countries.
Summary
JE is a neuroinvasive disease that causes symptoms ranging from simple fever to severe encephalitis and death. Despite a vast number of clinical trials on various drugs, there is still no complete cure available, and it can only be prevented by adequate vaccination. Various nanotechnological approaches for the prevention and treatment of JE are outlined in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese encephalitis (JE) is one of the most diagnosed endemic encephalitis infections in the world. It causes severe inflammation in the central nervous system (CNS) and mainly affects children below 6 years of age in endemic areas and both children and adults in non-endemic areas [1]. The fatality rate of JE is around 25 to 30%, and about 30 to 50% of JE survivors suffer long-term neurological consequences, posing a significant burden on public health and society [2]. The first case of JE was reported in 1871, and during a severe outbreak in Japan in 1924, a filterable agent was extracted from the human brain and transmitted to rabbits, although the agent was unknown at the time of identification [1, 3]. There is no antiviral treatment or other drugs available, which has been proven to completely cure the JE virus (JEV) infection. JEV is spread by mosquitoes, and Culex mosquito is the main vector in most endemic locations [4]. Since the mosquitoes that carry the JE virus thrive in rice paddies and other open water sources, hence vector control techniques are highly inefficient. According to the World Health Organization (WHO), human vaccination is the most effective tool for prevention of JE, and the JE vaccine should be included in immunization programmes at all places where JE is a public health issue [5].
Epidemiology
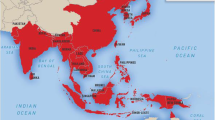
JE is mainly prevalent in the southern and south-eastern parts of Asia and the Pacific Rim. According to the WHO, JEV transmission is endemic in 24 countries in the West Asia and Western Pacific areas, putting more than 3 billion people at risk of infection. Nearly 68,000 cases of JE occur annually with an incidence of more than 10 in 100,000 population during outbreaks resulting in 13,600 to 20,400 deaths [6]. Seasonal patterns with rare outbreaks can be seen in the north (Bangladesh, Bhutan, China, Japan, South Korea, North Korea, Nepal, northern Vietnam, northern India, northern Thailand, Pakistan and Russia), whereas in the south (Australia, Burma, Brunei Darussalam, Cambodia, Indonesia, Malaysia, Philippines, Singapore, southern Vietnam, southern Thailand, southern India and Sri Lanka), endemic patterns are observed intermittently throughout the year [7]. The geographical distribution of JE is shown in Fig. 1.
Geographical distribution of Japanese encephalitis (reproduced with permission from Sharma et al. [8])
The risk of JE for most Asian visitors is quite low, although it varies according to the vacation destination, duration, season, activities and accommodation [9]. The total number of non-endemic travellers to Asia who contract JE is estimated to be fewer than one case per million tourists. People who stay longer in rural areas where the JE virus is prevalent may have a risk level like the vulnerable local population. Travellers on short journeys may be more susceptible if they spend a lot of time outside in rural regions during active transmission times [10, 11].
JE has been a serious paediatric health problem since 1955, with outbreaks documented in several parts of India [3]. It has been actively evident in nearly every state of India, and in states like Uttar Pradesh, Bihar, Haryana, Maharashtra, Karnataka, Manipur, Tamil Nadu, Odisha, Andhra Pradesh, Assam, West Bengal and Kerala, JE is occurring regularly while Uttar Pradesh being the major zone for the occurrence of JE in India [12•]. Gorakhpur District of Uttar Pradesh and neighbouring districts have been witnessing seasonal outbreaks of acute encephalitis syndrome (AES) for the last three decades. Between 2004 and 2013, Murhekar et al. [13] documented JE outbreaks throughout the monsoon and post-monsoon months (June–October), with a significant death rate in children. The highest number of cases, i.e. more than 5737 cases, was reported in 2005 in Gorakhpur alone.
Japanese Encephalitis Virus
In 1935, the prototype strain of JEV, known as the Nakayama strain, was isolated from the human brain in Tokyo, Japan [14]. JE is caused by the JEV belonging to the family Flaviviridae and the genus Flavivirus. It belongs to a class of Arbovirus, which requires an arthropod for its transmission. It is closely related to viruses belonging to the same family as West Nile virus, St. Louis encephalitis virus, Zika virus and dengue virus. The phylogenetic hierarchy of JEV is listed in Table 1.
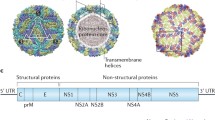
JEV is the most common Flavivirus-based mosquito-borne encephalitis infectious agent in the world [15]. It is a single-stranded positive sense RNA virus with a diameter of 40–50 nm and has a spheroid shape with cubical symmetry [16]. The cryo-electron microscopy study by Wang et al. [17•] showed that the virion consists of a central nucleocapsid core (viral RNA) and capsid protein. The JEV genome consists of 11 kB of viral RNA with an open reading frame flanked by a 5′ cap and a 3′ poly tail [18]. There are 2 types of protein in the genome of the virus: structural and non-structural. The structural proteins start from the 5′ end and include capsid (C), pre-membrane protein (prM) and envelope (E). The non-structural proteins (NS 1, 2A, 2B, 3, 4A, 4B and 5) take part in replication [19•]. The structure and genetic organization of JEV is shown in Fig. 2.
Structure and genomic arrangement of Japanese encephalitis virus (reproduced with permission from Unni et al. [20])
JEV is categorized into five genotypes (I, II, III, IV and V) based on the nucleotide sequence of the partial or complete viral genome [20]. Genotype III is mainly widespread in Asia until 1990, but now genotype I is also detected commonly [21]. Various strains of JEV based on the coding of prM and E protein coding genes are listed in Table 2 and Table 3, respectively.
Transmission Cycle of Japanese Encephalitis Virus
The JEV is transmitted to humans through the bite of infected Culex mosquitoes, mainly Culex tritaeniorhynchus, Culex tarsalis and Culex annulirostris [22]. The zoonotic transmission cycle and different stages involved in replication of JEV are shown in Figs. 3 and 4, respectively. The virus is spread by a zoonotic cycle of mosquitoes and vertebrate hosts, mostly pigs and wading birds (herons, egrets, etc.). Humans are unintended or dead-end hosts because they do not produce enough JE viraemia in their bloodstreams to infect feeding mosquitoes. Wading birds act as maintenance hosts in the transmission cycle of JEV whereas pigs play the most important part in the cycle as amplification host because of their higher capacity to produce JE viraemia, the higher titre of viraemia lasting up to 2–4 days to infect the mosquitoes [1].
Schematic representation of various steps involved in entry, replication, maturation and release of JEV from the host cells representing: 1 interaction of JEV with host cell receptor, 2 receptor-mediated endocytosis of virus, 3 fusion of viral and host cell membrane, 4 release of the viral genome into the cytoplasm, 5 formation of replication complex (RC) and cyclization of JEV genome, 6 formation of double-stranded replicative form (dsRF), 7 synthesis of viral genome in a semi-conservative and asymmetric manner, 8 translation and processing of JEV genome producing the 3 structural and 7 non-structural proteins, 9 JEV proteins associate with the RC and further assist viral replication, 10 maturation of virions in the Golgi complex and 11 release of mature virus (reproduced with permission from Unni et al. [20])
Infection in Humans
Most JE infections in humans are asymptomatic, while symptomatic infections are limited to specific locations. Asymptomatic JEV infection produces only immunoglobulin M (IgM) in the serum but no antibodies in the cerebrospinal fluid (CSF) [23]. The ratio of symptomatic to asymptomatic cases ranges from 1:25 to 1:1000 [24]. There are 3 stages of symptomatic JEV infection: (a) prodromal stage (headache, fever and malaise), (b) acute encephalitic stage (prominent symptoms such as muscular rigidity, convulsions, focal CNS signs, altered sensorium and coma) and (c) sequelae (neurologic signs stabilize or improve, resulting in recovery). JE is diagnosed by detecting the presence of JEV-specific IgM in the CSF of a patient by enzyme-linked immunosorbent assay [25–27].
Clinical Features of Japanese Encephalitis
The symptoms of JE infections develop in less than 1% of affected patients. Patients suffering from encephalitis are at risk of experiencing seizures during the acute stage as well as in the later stages. The infection of JE begins with a non-specific febrile illness, and the incubation period of JEV in humans is 5–15 days. Non-specific symptoms such as diarrhoea, coryza and rigors usually develop 3–4 days before the onset of AES, which manifests as clouding of consciousness, headache, vomiting and, in some cases, seizures [19•]. Electrophysiological experiments performed by Solomon et al. [23] showed that JEV myelitis is produced by injury to the anterior horn cells of the spinal cord, as in poliomyelitis. JE has also been associated with an autoimmune neuronal disorder known as Guillain–Barré syndrome. It causes demyelination and axonal damage, and inflammatory infiltrates of lymphocytes and monocytes have also been detected in a fatal case [28, 29].
Typical symptoms include a phase of fever, headache and vomiting lasting less than a week, followed by convulsions, coma and neurologic abnormalities with or without signs of meningeal irritation. Severe cases may result in a life-threatening surge in intracranial tension, decerebration or flaccid coma. This stage typically lasts for 7 to 10 days, after which there is progressive recovery with or without sequelae [30]. Further, severe dystonia and unusual movements (head nodding, lip-smacking, face grimacing, pill-rolling motions or choreoathetosis) may occur, gradually improving over weeks to months. In dire situations, hyperventilation, indications of increased intracranial tension, shock and death may happen progressively. Gastric haemorrhage is a major cause of mortality in critically sick children [31].
Treatment and Management of Japanese Encephalitis
Given the potential severity of the disease and its ability to spread in non-endemic areas, the lack of research in the treatment options for JE is a serious concern. An effective treatment is yet to be developed for the cure of JE. However, supportive treatment is beneficial in JE patients and certain clinical problems that elevate the risk of death are controllable [19•]. In case of seizures, the increase in intracranial pressure can be mitigated by the use of mannitol [32, 33]. Special care should be taken to avoid underhydration and overhydration of patients during illness [34]. In unconscious patients, practitioners should be on the watch for aspiration pneumonia, which must be treated as soon as clinical suspicion appears. In order to find a cure against JE, clinical trials have been conducted on various drugs like dexamethasone, minocycline and ribavirin which have been discussed in the following subsections [35].
Dexamethasone
Dexamethasone is a steroidal drug that was investigated as a potential treatment for JE. A study was carried out in southern Thailand during an epidemic season of JE in 1986. It consisted of 13 subjects with confirmed JE cases, in which 6 patients were treated with dexamethasone (with an initial loading dose of 0.6 mg/kg followed by 0.2 mg/kg every 6 h for 5 days) and 7 patients were treated as control group. Both the groups were examined daily for 5 days. It was reported that 2 patients treated with dexamethasone and 4 patients without any treatment died. The overall results of the clinical or laboratory measurements showed no remarkable change between the treatment and non-treatment pools. However, 13 patients were a too small sample to draw any type of conclusion [25].
In JE infection, it was observed that the death of patients occurred within 5 days of hospitalization due to an increase in intracranial pressure, which possibly caused depression and eventually death. Considering this, dexamethasone was investigated as a potential drug to alleviate the intracranial pressure. The trial included 55 individuals with confirmed JE cases, 25 of whom were administered dexamethasone and 30 were given a placebo. It was observed that dexamethasone did not bring about any difference in parameters like mortality rate and number of days hospitalized [36]. These studies suggested that dexamethasone could not provide any significant benefits in the treatment of JE in the clinical trials.
Minocycline
Minocycline is a semi-synthetic second-generation tetracycline that can cross the BBB and enter the CSF due to its small size (457.5 Da) and high lipophilicity. The neuroprotective and anti-inflammatory activity of minocycline has sparked great interest to explore minocycline in neurogenerative disorders and viral CNS infections [37]. In a study by Singh et al. [37], 44 patients who were IgM positive for JE were divided into two equal groups. One group was administered minocycline (5–6 mg/kg), and the other group was given a placebo as a control. It was observed that the group treated with minocycline showed beneficial effects like reduced fever, and mean duration of hospitalization which may be attributed to the anti-inflammatory and neuroprotective activities of minocycline [37].
In another study carried out by Kumar et al. [38] in Uttar Pradesh, India, out of 281 participating patients, 140 patients were administered nasogastric and oral minocycline and the remaining 141 patients were given a placebo. It was observed that minocycline showed beneficial results in patients older than 12 years of age and in patients who did not die very soon after being hospitalized. However, there were some problems in the study as the test was carried out in the poorest regions of India, which compromised the study due to the lack of proper resources and some patients included in the test were in dying condition [38]. Therefore, it can be concluded that minocycline could act as a potential anti-JE drug and further trials are required to be carried out taking a larger pool of subjects.
Ribavirin
Ribavirin is a broad-spectrum antiviral agent primarily used for the treatment of hepatitis C and viral haemorrhagic fevers. It is a synthetic guanosine analogue and a nucleoside inhibitor and acts by interfering with the synthesis of viral mRNA. As JEV is a single-stranded RNA virus, ribavirin may be used as a potential drug against it. A study was carried out between 2005 and 2007 in Gorakhpur, India, involving 153 children of 6–15 years of age, who were tested for the presence of IgM against JE and the presence of acute febrile encephalopathy. Out of 153 subjects, 70 received nasogastric or oral ribavirin (10 mg/kg divided into 4 doses per day for 7 days), and the other group of 83 subjects received placebo. After completion of the study, it was observed that ribavirin was ineffective against early mortality in children occurring due to JE [26]. Therefore, it can be concluded that though ribavirin has broad-spectrum antiviral action, it was ineffective to show any type of activity against JEV and is not recommended to be used for the treatment of JE.
Immunoglobulins
Intravenous immunoglobulins (IVIG) are postulated as a potential treatment for Flavivirus-related encephalitis (JEV, West Nile virus) because of their antiviral and anti-inflammatory properties. Rayamajhi et al. [39] conducted a study in Nepal involving 22 children out of which 13 were confirmed JE cases, divided into two groups of 11 each. They administered IVIG containing anti-JEV neutralizing antibody (400 mg/kg per day) to one group for 5 days, and the other group received 0.9% normal saline solution as a placebo. They observed that the group treated with IVIG exhibited higher titres (16-fold) of JEV-specific neutralizing antibody than the group treated with placebo. An increase in interleukin-4 and interleukin-6 levels was also observed in IVIG-treated patients [39]. Based on the above results, IVIG could be considered to be used as a potential treatment against JE, but the number of subjects in the above study was too low to derive any type of significant conclusion. Hence, further studies are required to be conducted with a larger number of subjects.
Interferon
Interferon (IFN)-α is a glycoprotein cytokine produced naturally as a part of innate immune response to viral infections. It induces the production of effector proteins in cells causing inhibition of viral replication, assembly and release. In a randomized double-blind trial carried out in Vietnam by Solomon et al. [40] involving 112 children with 87 serologically confirmed infections, 59 children were administered IFN-α 2a (10 million units/m2, daily) for 7 days and the rest were given a placebo. The researchers reported that IFN-α 2a was ineffective in JE patients since 21 children died and 17 had major sequelae. The researchers concluded that IFN-α 2a was not effective in JE patients when administered in this regimen since the 3-month outcome showed no significant difference [40]. Based on the findings, the authors recommended to carry out further research utilizing higher doses of IFN-α 2a administered via different routes, to determine the efficacy of the drug.
Natural Extracts
Numerous drugs obtained from natural sources have been explored for the prevention and treatment of various viral infections. Guo et al. [41] used a high content screening assay on 1034 natural extracts containing untapped reservoirs of potent and novel inhibitors and activators of various biological pathways to find a potential treatment of JEV infection. From the lot, ouabain and digoxin, two FDA-approved Na+/K+ ATPase inhibitors, were identified to have potential activities in the treatment of JE. The studies showed that both the drugs inhibited JEV infection at the replication stage by targeting Na+/K+ ATPase [29]. In the BALB/c mouse model, ouabain exhibited a significant reduction in morbidity and mortality by lowering the viral load and reducing degenerative brain damage whereas digoxin did not show any significant effect [41]. These findings suggested that ouabain has a significant anti-JE action, and the potential benefits should be investigated further.
It was observed that the drugs under investigation did not show any significant beneficial action in the treatment of JE. Hence, further research is required to find potential drugs for the effective treatment of JE. For now, vaccination remains the only way of protection against JEV infection.
Vaccines for Japanese Encephalitis
The most effective method for the prevention of JE to date is vaccination. The WHO recommends that the JE vaccine should be included in national immunization schedules in all regions where JE is a public health concern? Vaccination should be considered even in places where the number of JE-confirmed cases is moderate, as there is a risk of the propagation of JEV [42]. Randomized controlled trials have demonstrated the efficacy of vaccination against JE, as well as the relationship between neutralizing antibodies and protection [43, 44]. Different vaccines have been used to prevent JE since the 1950s, out of which early JE vaccines were primarily produced from mouse brains, but this production process has since been replaced by cell culture methods due to safety concerns as well as very poor immunogenic response in humans by the vaccines produced through the former approach [45]. There are currently four types of JE vaccines in use: mouse brain–derived inactivated vaccine, cell culture (Vero cell)–derived inactivated vaccine, cell culture–derived live attenuated JE vaccine (LAJEV) and live recombinant (chimeric) vaccine [5, 46]. The most widely produced and distributed vaccines were mouse brain inactivated type; however, these are no longer in use due to side effects such as acute disseminated encephalomyelitis [19•].
Types of Japanese Encephalitis Vaccines
There are currently various types of vaccines approved to be used in different countries for immunization against JE. The four major types of vaccines in use are mouse brain–derived inactivated vaccine (obtained from formalin-inactivated virus found in the brain of mouse), cell culture–derived inactivated vaccine (derived from Beijing-3 or Beijing-P3 strains of JEV cultivated in primary hamster kidney cells (PHKC)), live attenuated vaccine (obtained from attenuated SA14 strain of JEV) and live recombinant (chimeric) vaccine (developed by recombination of live attenuated JE vaccine and yellow fever vaccine) [47].
Mouse Brain–Derived Inactivated Japanese Encephalitis Vaccine
It was the first JE vaccine developed from a mouse brain–derived formalin-inactivated virus in 1934 by bacteriologist Tenji Taniguchi at Osaka University’s Research Foundation for Microbial Diseases, popularly known as the BIKEN Foundation. It was approved by the USFDA to be marketed in the USA as JE-VAX. This vaccine is developed from two strains, i.e. Nakayama strain and Beijing I strain of JEV grown in suckling mouse brain with the Beijing strain having a higher cross-reactivity among the JEV strains [48•]. According to a case–control study in Thailand, the efficacy of the mouse brain–derived JE vaccine was reported to be 94.6% and the adjusted effectiveness was nearly 97.5% which suggested that extensive immunization of mouse brain–derived JE vaccine may greatly reduce the burden of JE in the affected areas [49]. Although this vaccine is safe and efficient, some typical adverse effects include erythema, oedema, soreness, malaise, fever, headache and dizziness [50]. It also has a high production cost which makes it inaccessible to the areas where the vaccine is mostly required such as rural areas. It was unable to offer long-term immunity, necessitating the development of advanced vaccines.
Cell Culture–Derived Inactivated Japanese Encephalitis Vaccine
An inactivated vaccine made in PHKC culture is being used in China since 1967. It is derived from the Beijing-P3 strain which has fewer adverse effects, and is convenient to manufacture [48•]. It was licensed by the USFDA to be marketed in the USA as IXIARO for the use in patients of 17 years of age or older, and in 2013, USFDA also approved this vaccine to be used in children at age ranging from 2 months to 16 years of age. It is the only vaccine approved for travellers [51]. It is available in India as JEEV (Biological E. Ltd., Hyderabad, India) produced with an Austrian collaboration [4].
In a clinical trial conducted on 1993 adult volunteers to evaluate the safety of IXIARO, on administration of two doses of IXIARO, most of the symptoms were local discomfort and soreness while headache, myalgia, fatigue and influenza-like sickness were also reported in 10% of cases. Fever was the most frequently reported systemic response in youngsters. Further, IXIARO causes anaphylaxis in some cases, and it may lead to hypersensitivity in some patients due to the presence of protamine sulphate [52]. Since IXIARO was approved after a trial of around 5000 patients, the likelihood of rare significant side effects cannot be ruled out. Post-licensure investigations and surveillance are being carried out to assess IXIARO’s safety in a larger population [53].
In a phase 3, safety trial carried out in Philippines by Dubischar et al. [54] on 1869 children between the age group of 2 months and 17 years, the safety of IXIARO was compared with two of the standard vaccines, i.e. Prevnar (for children below 1 year of age) and HAVRIX 720 (for children aged 1 year and above). They observed that the occurrence of adverse effects was most frequent in children below 1 year of age, and it gradually decreased with age [54]. JENVAC is another Vero cell–derived purified inactivated JE vaccine that has acquired manufacturing and marketing approval from the Drug Controller General of India. This vaccine is developed by Bharat Biotech Ltd. in collaboration with the Indian Council of Medical Research (ICMR), New Delhi, India [4].
Live Attenuated Japanese Encephalitis Vaccine
The LAJEV was first approved in China in 1988 after initial trials showed no signs of adverse reactions. It is the most widely used vaccine in the JE-affected countries in Asia. China developed the SA14-14–2 live attenuated vaccine by transmitting SA14 strain of JEV to the PHKC [48•]. The vaccine causes attenuation by altering six amino acids in the E protein and three amino acids in the non-structural genes [55]. For children aged 9 months or older, a single dose of LAJEV is sufficient to protect them against clinical JE for at least 5 years [56]. In studies with single LAJEV dose, local symptoms such as erythema, oedema and discomfort at the injection site are commonly reported. Systemic reactions like cough, vomiting, rhinorrhoea, diarrhoea, drowsiness and fever (> 38 °C) were recovered within 1–2 days [57–60].
The frequency of JE cases and deaths has declined substantially in India and Nepal, with the implementation of LAJEV. The AES and JE lab monitoring systems in both the countries have improved along with the public awareness of JE disease prevention through vaccination [61]. In a study carried out in 2009, in Uttar Pradesh, India, the efficacy of a single shot of this vaccine was reported to be 94.5% after 6 months of vaccination [62]. Neighbouring countries have also taken efforts to design vaccination strategies of LAJEV for immunization against JE. LAJEV was one of the most widely used type of vaccines due to its higher efficacy, lesser side effects and lower cost of administration, but the main disadvantage of LAJEV is the process of preparing the vaccine, i.e. use of PHKC substrate, which is not approved by WHO for human vaccine development.
Live Recombinant (Chimeric) Japanese Encephalitis Vaccine
A chimeric vaccine is developed by substitution of genes of the target pathogens with the genes of a closely related organism which is safe. Sanofi Pasteur employed recombinant DNA techniques to develop a live attenuated chimeric viral vaccine called ‘IMOJEV’ in Vero cells by substituting the prM and E coding regions of the yellow fever live attenuated 17D virus with those of the SA14-14–2 LAJEV virus. In clinical trials carried out in Thailand, the seroconversion rates of this vaccine were 94% and 98.6% in phase II and phase III trials, respectively. In the children who took part in the trial, the JE chimeric vaccine was found to be safe and well-tolerated. Estimates of reactogenicity and other safety endpoints were lower in the JE chimeric vaccination group than in the SA14-14–2 vaccine group, but they remained within the same range [4, 63].
The initial document detailing the laboratory development of the chimeric vaccine by ChimerVax technology indicated that the structural proteins prM and E were mainly responsible for the attenuated phenotype of SA14-14–2 as well as chimeric vaccine. Notably, the chimeric vaccine strain showed eight amino acid alterations in the viral E glycoprotein compared to the parent virulent strain SA14. The chimeric virus expressing the prM-E of the wild-type Nakayama strain on the yellow fever 17D backbone exhibited neurovirulence and killed intracerebrally inoculated mice; however, when administered intracranially to Institute of Cancer Research (ICR) mice, the chimeric vaccine had entirely attenuated the neurovirulence [64]. Subcutaneous administration of this vaccine to non-human primates showed increased efficacy against the intracerebral effect of JE with a higher safety profile of the vaccine [65].
Recommendations for Administration of Japanese Encephalitis Vaccines
According to the current epidemiologic research, the risks of JE are changing and increasing worldwide. JE is a fluctuating and unpredictable hazard to travellers; however, safe and effective ways of immunization are available. Vaccines are advised to be administered to children in all areas as well as adults in the JE endemic areas. Vaccination is recommended to all immigrants residing in endemic countries, as well as travellers visiting rural or peri-urban regions in endemic nations, regardless of length or purpose of stay. Travellers who have unknown plans or plans that are subject to change should also be properly vaccinated [66]. The decision to vaccinate should be based on various factors like (a) hazards linked with a specific travel schedule; (b) morbidity and fatality rate of JE in the area; (c) access to an effective immunization; (d) whether there is a risk, albeit a low probability, of major adverse outcomes following immunization; and (e) probability of future travel to JE-endemic nations, etc. [67•]. The following are the vaccination schedules recommended by the WHO [42, 68].
-
a)
Live attenuated vaccine: single dose to be administered before or at 8 months of age
-
b)
Live chimeric vaccine: single dose to be administered before or at 9 months of age
-
c)
Inactivated vaccines: two doses to be administered in which the primary dose should be given before or at 6 months of age followed by a secondary dose after an interval of usually 4 weeks.
Booster doses have also been recommended based on the type of vaccine administered to further strengthen the immunity against JEV. JE vaccination is normally not suggested during pregnancy because of the danger to the developing fetus; however, vaccination is recommended if travelling to a JE-endemic region since the benefits of vaccination outweigh the risks to both the mother and the developing fetus [67•]. The list of various vaccines approved in different countries for immunization against JE is enlisted in Table 4.
Prevention and Control of Japanese Encephalitis
Only Asia and the Western Pacific have been identified to have local transmission of JEV to date, as a result, worldwide awareness of this terrible infection is limited. Large outbreaks occasionally create momentary excitement, but the disease and significant advances in its control have garnered little publicity. Japan, South Korea and Thailand have implemented effective JE vaccination programs that have significantly lowered the disease burden. Other nations, such as China, India, and Nepal, have also made significant strides in recent years [69–71]. Public health and veterinary authorities should be cautious to avoid spread of JEV [72]. Although vaccination is the most effective way of control and prevention against JE, some preventive measures can also help, especially in the endemic areas. As JE is spread by mosquito vectors, efforts should be made to control the mosquito populations in the endemic areas. The other method of preventing the spread of JE is effective management of cattle herding and human dwelling sites.
Vector Control
The major cause of the spread of JEV is vector transmission of the virus by mosquito C. tritaeniorhynchus which breeds in the pools of stagnant water like paddy fields or drainage ditches. Although vector control can be used to mitigate short-term outbreaks in endemic areas, this method of prevention has limited efficacy and is also considerably expensive in most cases. Insecticides can be employed; however, resistance in mosquito vectors is a major drawback and will render long-term treatment ineffective. Another technique is to apply larvicides to rice crops. The natural pesticide “neem” sprayed on rice fields, as well as the placement of larval fish in rice paddies, could be more ecological approach. In Assam, northeast India, insecticide-treated mosquito nets were employed to evaluate the effect of JEV seroconversion in pigs and people. Following the intervention, there was a significant reduction in the rate of seroconversion in pigs and people [4].
Cattle are dead-end hosts for JEV; therefore, using cattle to deflect mosquitoes away from pigs and people might be one solution (zooprophylaxis). Long-term methods to avoid mosquitoes include better rural water management, intermittent watering of rice fields to disrupt mosquito reproduction without reducing rice productivity and adequate personal protection against mosquito bites, such as the use of mosquito nets, insect repellents and protective clothing that are also important supportive measures to control this disease.
Measures Against Vertebrate Hosts and Humans
Efforts should be made to keep the pig population away from mosquito-borne areas and to control the mosquito population where pigs are being reared at a larger scale. Pig farms should be built away from human dwelling areas and steps should be taken to immunize pigs and cattle in the endemic areas. In case of humans, vaccination is considered to be the best prevention measure against JE, but other precautions are important because access to vaccines is still not available in many underdeveloped countries of the world. Various measures to reduce human mosquito exposure, such as repellents, protective clothing, window screens, conditioning systems and behavioural changes, may be useful for short-term visitors or urban residents, but given the extent and duration of exposure, they are inadequate in most rural areas where it is endemic [73].
Many JE control actions and collaborations were pioneered by the Program for Appropriate Technology in Health JE project, which was funded by the Bill and Melinda Gates Foundation. The control of JE is acknowledged as a key factor for achieving the Millennium Development Goals, and it was supported by a World Health Assembly resolution on disability in 2005 [74]. Urban development, better socioeconomic conditions and agricultural advancements have all contributed to a significant reduction in the burden of JE in many Asian countries. Programs should be developed to raise public knowledge about the illness, and vaccination should be properly managed to ensure that vaccines are available to everyone with the least amount of inconvenience in order to effectively reduce the effects of JE. In the endemic areas, the general population should be educated about the disease and ways to prevent it.
Nanotechnology in Japanese Encephalitis
Nanotechnology is an innovative approach in pharmaceutical and biomedical field which can overcome many hurdles faced by the conventional drug delivery systems including low bioavailability and peripheral toxicities of drugs owing to the various unique characteristics like nano size range, excellent targeting ability and enhanced cellular uptake. Various approaches using different nanocarriers like nanoparticles, nanoemulsions, microemulsions, quantum dots, dendrimers and so on have been employed for the treatment of infectious diseases [75–78]. Nanotechnology is also widely employed in the development of diagnostic tools such as electrochemical biosensor associated metallic nanoparticles for the rapid detection of various mosquito-borne diseases like JE, dengue and yellow fever virus [79, 80].
Various nanotechnology-based methods are being employed to find a potential treatment against JE, especially from natural sources. Antiviral properties of natural products combined with the advantages brought about by nanocarriers can synergistically aid in the treatment of JE. Chen et al. [81•] developed curcumin carbon quantum dots (Cur-CQD) using mild pyrolysis polymerization and carbonization for targeting the E protein of JEV. The synthesis of Cur-CQD significantly increased the water solubility and reduced the cytotoxicity of curcumin by 10 times making it a superior antiviral agent against JEV. Transmission electron microscopy, biolayer interferometry and molecular docking analysis proved that mutations in S123R and K312R sequences in E protein are crucial for the binding of Cur-CQD. The time-of-addition assay in BHK-21, HEK-293 T and Vero cell lines showed that Cur-CQD inhibited the early infection stage of JEV. They suggested that combined administration of antiviral drugs along with curcumin-based nanomaterials could provide synergistic antiviral effect and help in the inhibition of viral infection [81•]. Nanocarrier systems targeting various regions of the viral RNA can drastically limit the activity of JEV and can be further researched to develop an effective treatment against JE.
Vector control is one of the most important aspects in limiting the spread of JEV and many such deadly infections. Nanotechnology-based vector control systems are extensively researched to control the vector populations and limit the spread of such infections. Govindarajan et al. [82] synthesized silver nanoparticles with aqueous leaf extract of Anisomeles indica and investigated their mosquitocidal potential against malaria, dengue and JE vectors. The nanoparticles were of the size 25 nm with spherical shape as observed in transmission electron microscope images. Larvicidal activity of A. indica leaf extract and the silver nanoparticles evaluated on Anopheles subpictus, Aedes albopictus and C. tritaeniorhynchus larvae showed dose-dependent toxicity. The silver nanoparticles exhibited higher toxicity than the leaf extract in A. subpictus, A. albopictus and C. tritaeniorhynchus with LC50 values of 31.56 μg/mL, 35.21 μg/mL and 38.08 μg/mL, respectively. Nanoformulations based on A. indica may be further explored for the development of newer and safer larvicides for the control of various mosquito-borne infections including malaria, dengue and JE [82].
In another study, Gupta et al. [83•] prepared thyme oil nanoemulsion using high-energy method and encapsulated it in chitosan for the control of various mosquito-borne infections. Thermodynamic stability studies of the coated and uncoated nanoemulsions displayed that 1:0.5 (oil:Tween 80) and 1:1 (nanoemulsion:chitosan solution) ratios showed the maximum stability. The particle size of the uncoated and coated nanoemulsions was found to be 52.18 ± 4.53 nm and 50.01 ± 2.32 nm, respectively, with a narrow polydispersity index in dynamic light scattering studies. Transmission electron microscopy showed that the size of uncoated nanoemulsion ranged from 40 to 110 nm and that of coated nanoemulsion was 76–126 nm with a spherical shape. In vitro release studies demonstrated the sustained release behaviour of both the formulations with 91.68% and 73.41% release of thyme oil in 48 h from uncoated and coated nanoemulsions, respectively. Larvicidal activity of the prepared formulations was evaluated against III instar larvae of Anopheles stephensi, Aedes aegypti and C. tritaeniorhynchus. After 24 h of exposure, the uncoated nanoemulsion showed the highest activity against C. tritaeniorhynchus with LC50 of 22.58 ppm, whereas the coated nanoemulsion showed maximum activity against A. stephensi with LC50 of 18.88 ppm [83•].
These studies suggested the potential larvicidal activities of the nanotechnology-based formulations against III instar larvae of various mosquito species. Further, nanocarriers based on plant derivatives and essential oils could be researched for development of newer and safer insecticides to effectively control the vector populations of various infectious diseases.
Lack of successful treatment strategies to effectively cure JE makes vaccines the most ideal choice for protection against JE. Currently available vaccination regimens involve invasive approaches resulting in patient incompliance especially in the case of children. This limitation calls for development of non-invasive methods to effectively deliver the vaccines. Huang et al. [84] developed chitosan nanoparticle–based DNA vaccine, pCJ-3/ME against JE for non-invasive transdermal vaccine immunization utilizing a low-pressure gene gun. The transdermal delivery efficiency of the vaccine was tested on female C3H/HeN mice, and the results showed that chitosan nanoparticles had the ability to transport DNAs to specific areas via transdermal route by jet-propelling of a low-pressure gene gun. The chitosan/pCJ-3/ME complex was applied topically to the mice, and the complex could elicit efficient humoral immunity against JEV’s lethal dose. Further, the chitosan/pCJ-3/ME complex with the ImmunEasy™ CpG mouse adjuvants showed a synergistic effect with a good correlation between the degrees of immunogenicity and the provoked antibodies. Authors reported that chitosan-based JEV DNA vaccine delivered via low-pressure gene gun showed higher efficacy against JEV and concluded that it can be explored for future prophylaxis against JEV outbreaks [84].
Such non-invasive methods of vaccinations can be researched further for effective immunization against JEV and other infectious diseases.
Conclusion
JE is a zoonotic disease caused by JEV with a natural life cycle in pigs, birds and mosquitoes, with humans serving as an unintentional dead-end host. It regularly causes epidemics and breakouts in Asia, particularly affecting impoverished rural rice farmers and pig rearers. It mainly affects the children below 6 years of age with serious symptoms and, if not properly treated, may even result in death. Specific treatment strategies, which are proven to be effective to completely cure JE, have not been developed yet. Many drugs have also gone through clinical trials but have often failed to provide promising results.
JE poses to be a matter of concern in many Asian countries and some parts of the world despite the availability of effective vaccines. This may be due to improper diagnosis, improper storage of vaccines, inaccessibility to remote areas and the emergence of new viral strains. Since JE is a worldwide problem, so multicentre epidemiology and post-vaccination monitoring studies are required.
Though JE research has advanced significantly in recent years in terms of understanding the viral structure, disease pathogenesis and so on, there is still a large opportunity for clinical research and the development of more sensitive diagnostic tools to detect and prevent the disease in its early stages. Considering the severity of the disease JE, comprehensive research investigations are required to be carried out systematically to improve control, prevention, diagnosis and treatment of this worldwide problem, which is endemic to certain parts of the world.
Abbreviations
- AES:
-
Acute encephalitis syndrome
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- IgM:
-
Immunoglobulin M
- INF:
-
Interferon
- IVIG:
-
Intravenous immunoglobulins
- JE:
-
Japanese encephalitis
- JEV:
-
Japanese encephalitis virus
- LAJEV:
-
Live attenuated JE vaccine
- PHKC:
-
Primary hamster kidney cells
- WHO:
-
World health organization
References
Papers of particular interest, published recently, have been highlighted as: • of importance
Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–20.
Banerjee A, Tripathi A. Recent advances in understanding Japanese encephalitis. F1000Res. 2019;8. https://pubmed.ncbi.nlm.nih.gov/31781366/.
Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis Centers for Disease Control and Prevention. 2009;15:1. /pmc/articles/PMC2660690/.
Kumar R. Prevention, diagnosis, and management of Japanese encephalitis in children. Pediatr Heal Med Ther. 2014;5:99.
Hills S, Martin R, Marfin A, Fischer M. Control of Japanese encephalitis in Asia: the time is now. Expert Rev Anti Infect Ther. NIH Public Access; 2014;12:901. /pmc/articles/PMC4594829/.
Japanese encephalitis. https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis.
Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag. 2015;11:435–48. https://pubmed.ncbi.nlm.nih.gov/25848290/.
Sharma KB, Vrati S, Kalia M. Pathobiology of Japanese encephalitis virus infection. Mol Aspects Med. 2021;81.
Japanese encephalitis - Chapter 4 - 2020 yellow book | Travelers’ Health | CDC. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/japanese-encephalitis.
Wittesjö B, Eitrem R, Niklasson B, Vene S, Mangiafico JA. Japanese encephalitis after a 10-day holiday in Bali. Lancet. Elsevier. 1995;345:856.
Caramello P, Canta F, Balbiano R, Lipani F, Ariaudo S, De Agostini M, et al. A case of imported JE acquired during short travel in Vietnam. Are current recommendations about vaccination broader? J Travel Med Oxford Academic. 2007;14:346–8. https://academic.oup.com/jtm/article/14/5/346/1809058.
• Kulkarni R, Sapkal G, Kaushal H, Mourya D. Japanese encephalitis: a brief review on Indian perspectives. Open Virol J. 2018;12:121–30. https://pubmed.ncbi.nlm.nih.gov/30288200/. Reports the major JE endemic areas in India.
Murhekar M, Vivian Thangaraj J, Sadanandane C, Mittal M, Gupta N, Rose W, et al. Investigations of seasonal outbreaks of acute encephalitis syndrome due to Orientia tsutsugamushi in Gorakhpur region, India: a One Health case study. Indian J Med Res. 2021;153:375–81. https://pubmed.ncbi.nlm.nih.gov/33907001/.
Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ, Barrett ADT. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol Am Soc Microbiol. 2003;77:3091–8. https://journals.asm.org/journal/jvi.
Roberts A, Gandhi S. Japanese encephalitis virus: a review on emerging diagnostic techniques. Front Biosci (Landmark Ed); 2020;25:1875–93. https://pubmed.ncbi.nlm.nih.gov/32472762/.
K. Saxena S, Tiwari S, Saxena R, Mathur A, N. Nair MP. Japanese encephalitis virus: the complex biology of an emerging pathogen. Encephalitis. 2013.
• Wang X, Li SH, Zhu L, Nian QG, Yuan S, Gao Q, et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat Commun Springer, US. 2017;8:1–8. https://doi.org/10.1038/s41467-017-00024-6. Studied the structure of JEV through cryo-electron microscopy.
Vashist S, Bhullar D, Vrati S. La protein can simultaneously bind to both 3’- and 5’-noncoding regions of Japanese encephalitis virus genome. DNA Cell Biol. 2011;30:339–46. https://pubmed.ncbi.nlm.nih.gov/21294637/.
• Turtle L, Solomon T. Japanese encephalitis-the prospects for new treatments. Nat Rev Neurol. 2018;14:298–313. Described the genetic structure of RNA in JEV.
Unni SK, Růžek D, Chhatbar C, Mishra R, Johri MK, Singh SK. Japanese encephalitis virus: from genome to infectome. Microbes Infect. 2011;13:312–21.
Seo HJ, Kim HC, Klein TA, Ramey AM, Lee JH, Kyung SG, et al. Molecular detection and genotyping of Japanese encephalitis virus in mosquitoes during a 2010 outbreak in the Republic of Korea. PLoS ONE. 2013;8:1–11.
Hurk AF van den, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev of Entomology. 2008;54:17–35. https://doi.org/10.1146/annurev.ento.54.110807.090510.
Solomon T, Kneen R, Dung N, Khanh V, Thuy T, Ha D, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet (London, England). 1998;351:1094–7. https://pubmed.ncbi.nlm.nih.gov/9660579/.
Solomon T, Winter P. Neurovirulence and host factors in flavivirus encephalitis--evidence from clinical epidemiology. Arch Virol Suppl. 2004;161–70. https://pubmed.ncbi.nlm.nih.gov/15119771/.
Johnson RT, Intralawan P, Puapanwatton S. Japanese encephalitis: identification of inflammatory cells in cerebrospinal fluid. Ann Neurol. 1986;20:691–5.
Kumar R, Tripathi P, Baranwal M, Singh S, Tripathi S, Banerjee G. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh. India Clin Infect Dis. 2009;48:400–6.
Hills S, Dabbagh A, Jacobson J, Marfin A, Featherstone D, Hombach J, et al. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BioMed Central. 2009;9:214. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2809064/.
Wang G, Li H, Yang X, Guo T, Wang L, Zhao Z, et al. Guillain-Barré syndrome associated with JEV infection. N Engl J Med. 2020;383:1188–90. https://pubmed.ncbi.nlm.nih.gov/32937054/.
Ravi V, Taly AB, Shankar SK, Shenoy PK, Desai A, Nagaraja D, et al. Association of Japanese encephalitis virus infection with Guillain-Barré syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90:67–72. https://pubmed.ncbi.nlm.nih.gov/7941960/.
Kumar R. Understanding and managing acute encephalitis. F1000Res. 2020;9. https://pubmed.ncbi.nlm.nih.gov/32047620/.
Kumar R, Tripathi P, Singh S, Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43:123–31. https://pubmed.ncbi.nlm.nih.gov/16779737/.
Solomon T, Dung N, Kneen R, Thao le T, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–93. https://pubmed.ncbi.nlm.nih.gov/11960897/.
Tiwari S, Singh R, Tiwari R, Dhole T. Japanese encephalitis: a review of the Indian perspective. Braz J Infect Dis. 2012;16:564–73. https://pubmed.ncbi.nlm.nih.gov/23141974/.
Rayamajhi A, Ansari I, Ledger E, Bista KP, Impoinvil DE, Nightingale S, et al. Clinical and prognostic features among children with acute encephalitis syndrome in Nepal; a retrospective study. BioMed Central. 2011;11:1–12. https://bmcinfectdis.biomedcentral.com/articles/https://doi.org/10.1186/1471-2334-11-294.
Ajibowo AO, Ortiz JF, Alli A, Halan T, Kolawole OA. Management of Japanese encephalitis: a current update. Cureus. 2021;13.
Hoke CH, Vaughn DW, Nisalak A, Intralawan P, Poolsuppasit S, Jongsawas V, et al. Effect of high-dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis. 1992;165:631–7.
Singh DAK, Mehta DA, Kushwaha DKP, Pandey AK, Mittal DM, Sharma DB, et al. Minocycline trial in Japanese encephalitis: a double blind, randomized placebo study. Pediatr Rev Int J Pediatr Res. 2016;3:371–7. https://pediatrics.medresearch.in/index.php/ijpr/article/view/120/237.
Kumar R, Basu A, Sinha S, Das M, Tripathi P, Jain A, et al. Role of oral minocycline in acute encephalitis syndrome in India - a randomized controlled trial. BMC Infect Dis. 2016;16:1–10. https://doi.org/10.1186/s12879-016-1385-6.
Rayamajhi A, Nightingale S, Bhatta NK, Singh R, Ledger E, Bista KP, et al. A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal. PLoS ONE. 2015;10:1–21. https://doi.org/10.1371/journal.pone.0122608.
Solomon T, Dung NM, Wills B, Kneen R, Gainsborough M, Diet TV, et al. Interferon alfa-2a in Japanese encephalitis: a randomised double-blind placebo-controlled trial. Lancet. 2003;361:821–6.
Guo J, Jia X, Liu Y, Wang S, Cao J, Zhang B, et al. Screening of natural extracts for inhibitors against Japanese encephalitis virus infection. Antimicrob Agents Chemother. 2020;64:1–11.
Japanese encephalitis vaccines: WHO position paper - February 2015; 90:69–88. https://apps.who.int/iris/handle/10665/242325.
Dubischar K, Kadlecek V, Sablan B, Borja-Tabora C, Gatchalian S, Eder-Lingelbach S, et al. Safety of the inactivated Japanese encephalitis virus vaccine IXIARO in children: an open-label, randomized, active-controlled, phase 3 study. Pediatr Infect Dis J. 2017;36:889–97. https://pubmed.ncbi.nlm.nih.gov/28441266/.
Vadrevu K, Potula V, Khalatkar V, Mahantshetty N, Shah A, Ella R. Persistence of immune responses with an inactivated Japanese encephalitis single-dose vaccine, JENVAC and interchangeability with a live-attenuated vaccine. J Infect Dis. 2020;222:1478–87. https://pubmed.ncbi.nlm.nih.gov/31858116/.
Hegde N, Gore M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum Vaccin Immunother. 2017;13:1320–37. https://pubmed.ncbi.nlm.nih.gov/28301270/.
Batchelor P, Petersen K. Japanese encephalitis: a review of clinical guidelines and vaccine availability in Asia. Trop Dis Travel Med Vaccines BioMed Central. 2015;1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5530929/.
Satchidanandam V. Japanese encephalitis vaccines. Curr Treat Options Infect Dis. 2020;12:375–86. http://link.springer.com/https://doi.org/10.1007/s40506-020-00242-5.
• Kumar A, Sharma P, Shukla KK, Misra S, Nyati KK. Japanese encephalitis virus: associated immune response and recent progress in vaccine development. Microb Pathog. Elsevier Ltd. 2019;136:103678. https://doi.org/10.1016/j.micpath.2019.103678. This review povides a brief description on different JE vaccines.
Muangchana C, Henprasertthae N, Nurach K, Theppang K, Yoocharoen P, Varinsathien P, et al. Effectiveness of mouse brain-derived inactivated Japanese encephalitis vaccine in Thai National Immunization Program: a case-control study. Vaccine Elsevier Ltd. 2012;30:361–7. https://doi.org/10.1016/j.vaccine.2011.10.083.
Plesner AM. Allergic reactions to Japanese encephalitis vaccine. Immunol Allergy Clin North Am Elsevier. 2003;23:665–97.
IXIARO | FDA https://www.fda.gov/vaccines-blood-biologics/vaccines/ixiaro.
Food and Drug Administration. Ixiaro: Japanese encephalitis vaccine, inactivated, adsorbed [package insert]. Vienna, Austria: Valneva Austria GmbH; 2018 https://www.google.com/search?q=Food+and+Drug+Administration.+Ixiaro%3A+Japanese+encephalitis+vaccine%2C+inactivated%2C+adsorbed+%5Bpackage+insert%5D.+Vienna%2C+Austria%3A+Valneva+Austria+GmbH%3B+2018&rlz=1C1CHBF_enIN945IN945&oq=Food+and+Drug+Administrati.
Schuller E, Klingler A, Dubischar-Kastner K, Dewasthaly S, Müller Z. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO(®).Vaccine; 2011;29:8669–76. https://pubmed.ncbi.nlm.nih.gov/21907747/.
Dubischar KL, Kadlecek V, Sablan B, Borja-Tabora CF, Gatchalian S, Eder-Lingelbach S, et al. Safety of the inactivated Japanese encephalitis virus vaccine IXIARO in children: an open-label, randomized, active-controlled, phase 3 study. Pediatr. Infect. Dis. J. 2017.
Ni H, Burns NJ, Chang GJJ, Zhang MJ, Wills MR, Trent DW, et al. Comparison of nucleotide and deduced amino acid sequence of the 5’ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J Gen Virol. 1994;75:1505–10.
Yongxin Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28:3635–41.
Ranganath BG, Hiremath SG. Adverse events following immunisation with SA 14–14-2 Japanese encephalitis vaccine in children of Kolar in Karnataka. J Indian Med Assoc. 2012;110:10–2.
Chotpitayasunondh T, Sohn YM, Yoksan S, Min J, Ohrr H. Immunizing children aged 9 to 15 months with live attenuated SA14-14-2 Japanese encephalitis vaccine in Thailand. J Med Assoc Thai. 2011;94(Suppl):3.
Zaman K, Naser AM, Power M, Yaich M, Zhang L, Ginsburg AS, et al. Lot-to-lot consistency of live attenuated SA 14–14-2 Japanese encephalitis vaccine manufactured in a good manufacturing practice facility and non-inferiority with respect to an earlier product. Vaccine. 2014;32:6061–6.
Sanchayan K, Fernandopulle R, Amarasinghe A, Thiyahiny S, Sri Ranganathan S. Safety of live attenuated Japanese encephalitis vaccine given at the age of 9 months in National Immunisation Programme of Sri Lanka. Ceylon Med J. 2016;61:99–105. https://pubmed.ncbi.nlm.nih.gov/27727408/.
Upreti SR, Janusz KB, Schluter WW, Bichha RP, Shakya G, Biggerstaff BJ, et al. Estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA 14–14-2 vaccine in Nepal. Am J Trop Med Hyg. 2013;88:464–8.
Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14–14-2 vaccine against Japanese encephalitis. N Engl J Med. 2009;360:1465–6.
Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, et al. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14–14–2: a randomized study of immunogenicity and safety. Pediatr Infect Dis J. 2014;33:643–9. https://pubmed.ncbi.nlm.nih.gov/24717964/.
Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol. 1999;73:3095–101. https://pubmed.ncbi.nlm.nih.gov/10074160/.
Monath TP, Soike K, Levenbook I, Zhang ZX, Arroyo J, Delagrave S, et al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine. 1999;17:1869–82.
Connor B, Bunn WB. The changing epidemiology of Japanese encephalitis and new data: the implications for New recommendations for Japanese encephalitis vaccine. Trop Dis Travel Med Vaccines. 2017;3:1–6.
• Hills SL, Walter EB, Atmar RL, Fischer M. Japanese encephalitis vaccine: recommendations of the advisory committee on immunization practices. MMWR Recomm. Reports. 2019. This review outlines the recommendations for administration of JE vaccines.
Lee PI, Huang YC, Hwang KP, Liu CC, Chiu CH, Chen PY, et al. Recommendations for the use of Japanese encephalitis vaccines. Pediatr Neonatol. Elsevier Taiwan LLC; 2020;61:3–8. Available from: https://doi.org/10.1016/j.pedneo.2019.11.009.
Upreti SR, Lindsey NP, Bohara R, Choudhary GR, Shakya S, Gautam M, et al. Updated estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA 14–14–2 vaccine in Nepal. PLoS Negl Trop Dis Public Library of Science. 2017;11:e0005866. https://journals.plos.org/plosntds/article?id=https://doi.org/10.1371/journal.pntd.0005866.
Heffelfinger JD, Li X, Batmunkh N, Grabovac V, Diorditsa S, Liyanage JB, et al. Japanese encephalitis surveillance and immunization - Asia and Western Pacific regions, 2016. Centers for Disease Control MMWR Office. 2019;66:579–83. https://www.facebook.com/CDCMMWR.
Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89. https://pubmed.ncbi.nlm.nih.gov/22084515/.
Mansfield K, Hernández-Triana L, Banyard A, Fooks A, Johnson N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol. 2017;201:85–92. https://pubmed.ncbi.nlm.nih.gov/28284628/.
Fischer M, Hills S, Staples E, Johnson B, Yaich M, Solomon T. Japanese encephalitis prevention and control: advances, challenges, and new initiatives. Emerg Infect. 2014;8:93–124.
Morbidity JE. Accomplishments and lessons learned. Advocacy Japanese Enceph. 2009.
Jain K, Ahmad J, Rizwanullah M, Suthar T, Albarqi HA, Ahmad MZ, et al. Receptor-targeted surface engineered nanomaterials for breast cancer imaging and theranostic applications. Crit Rev Ther Drug Carr Syst. Begel House Inc.. 2022. https://www.dl.begellhouse.com/references/3667c4ae6e8fd136,forthcoming,40686.html.
Das B, Patra S. Antimicrobials: meeting the challenges of antibiotic resistance through nanotechnology. Nanostructures Antimicrob Ther Nanostructures Ther Med Ser. Elsevier. 2017;1–22.
Juneja M, Suthar T, Pardhi VP, Ahmad J, Jain K. Emerging trends and promises of nanoemulsions in therapeutics of infectious diseases. 2022.
Mba IE, Nweze EI. Application of nanotechnology in the treatment of infectious diseases: an overview. Nanotechnol Infect Dis Singapore: Springer, Singapore. 2022;25–51. https://doi.org/10.1007/978-981-16-9190-4_2.
Duarte JL, Filippo LD Di, Araujo VHS, Oliveira AEM de FM, de Araújo JTC, Silva FB da R, et al. Nanotechnology as a tool for detection and treatment of arbovirus infections. Acta Trop. 2021;216.
Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol. Elsevier. 2019;53:101174.
• Chen H-H, Lin C-J, Anand A, Lin H-J, Lin H-Y, Mao J-Y, et al. Development of antiviral carbon quantum dots that target the Japanese encephalitis virus envelope protein. J Biol Chem Am Soc Biochem Mol Biol. 2022;101957. Available from: https://doi.org/10.1016/j.jbc.2022.101957. Proposes the use of nanotechnology in the treatment of JE infection.
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Benelli G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp Parasitol. Elsevier Inc. 2016;161:40–7. https://doi.org/10.1016/j.exppara.2015.12.011.
• Gupta P, Preet S, Ananya, Singh N. Preparation of Thymus vulgaris (L.) essential oil nanoemulsion and its chitosan encapsulation for controlling mosquito vectors. Sci Rep. Nature Publishing Group UK. 2022;12:1–14. https://doi.org/10.1038/s41598-022-07676-5. Proposed nanotechnology-based method to effectively control the population of mosquito vectors in various infections.
Huang HN, Li TL, Chan YL, Chen CL, Wu CJ. Transdermal immunization with low-pressure-gene-gun mediated chitosan-based DNA vaccines against Japanese encephalitis virus. Biomaterials Elsevier Ltd. 2009;30:6017–25. https://doi.org/10.1016/j.biomaterials.2009.07.029.
Acknowledgements
The authors are grateful to the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India, for providing the facilities to write this manuscript. The NIPER-R communication number for this manuscript is NIPER-R/Communication/341.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Infectious Diseases
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahu, R.C., Suthar, T., Pathak, A. et al. Interventions for the Prevention and Treatment of Japanese Encephalitis. Curr Infect Dis Rep 24, 189–204 (2022). https://doi.org/10.1007/s11908-022-00786-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-022-00786-1