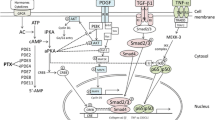
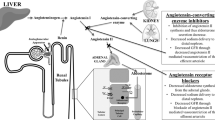
Abstract
Experimental and clinical studies have shown that blockade of the renin-angiotensin system (RAS) is effective in reducing proteinuria in conditions such as diabetes by reducing systemic and intraglomerular hydrostatic pressure. However, increasing evidence suggests that nonhemodynamic effects, such as preservation of the podocyte slit diaphragm structure and function, may also mediate the antiproteinuric effects of RAS blockade. In this review, we analyze in detail the evidence for known and novel mechanisms considered to play important roles in mediating the antiproteinuric effect of RAS blockers, with a particular focus on diabetic nephropathy.
Similar content being viewed by others
References and Recommended Reading
Rossing P: Promotion, prediction, and prevention of progression in diabetic nephropathy. Dan Med Bull 1998, 45:354–369.
Peterson JC, Adler S, Burkart JM, et al.: Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995, 123:754–762.
Keane WF, Brenner BM, de Zeeuw D, et al.: The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003, 63:1499–1507.
Borch-Johnsen K, Kreiner S: Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987, 294:1651–1654.
Gall MA, Borch-Johnsen K, Hougaard P, et al.: Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 1995, 44:1303–1309.
Apperloo AJ, de Zeeuw D, de Jong PE: Short-term antiproteinuric response to antihypertensive treatment predicts long-term GFR decline in patients with non-diabetic renal disease. Kidney Int Suppl 1994, 45:S174-S178.
Shahinfar S, Dickson TZ, Ahmed T, et al.: Losartan in patients with type 2 diabetes and proteinuria: observations from the RENAAL Study. Kidney Int Suppl 2002, 82:64–67.
Parving HH, Andersen S, Jacobsen P, et al.: Angiotensin receptor blockers in diabetic nephropathy: renal and cardiovascular end points. Semin Nephrol 2004, 24:147–157. Extensive review summarizing the recent evidence for the effects of angiotensin receptor blockers in diabetic nephropathy, with a particular focus on effects on proteinuria and translation into renal and cardiovascular end points.
De Zeeuw D, Remuzzi G, Parving HH, et al.: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004, 65:2309–2320. This post-hoc analysis emphasizes in detail the importance of baseline proteinuria and the effect of RAS blockade on proteinuria as a predictor of long-term renal protection and outcome.
Gilbert RE, Cooper ME: The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 1999, 56:1627–1637.
Abdi R, Brenner BM: Impact of renin angiotensin system blockade on renal function in health and disease: an end or a beginning? Semin Nephrol 2004, 24:141–146.
Doublier S, Salvidio G, Lupia E, et al.: Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 2003, 52:1023–1030. Some of the first evidence for nephrin depletion in human diabetic nephropathy and the association with angiotensin II.
Ruperez M, Ruiz-Ortega M, Esteban V, et al.: Angiotensin II increases connective tissue growth factor in the kidney. Am J Pathol 2003, 163:1937–1947.
Touyz RM, Schiffrin EL: Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 2001, 19:1245–1254.
Group AIiDNT: Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensinconverting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001, 134:370–379.
Lewis EJ, Hunsicker LG, Clarke WR, et al.: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001, 345:851–860.
Ravid M, Lang R, Rachmani R, Lishner M: Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus: a 7-year follow-up study. Arch Intern Med 1996, 156:286–289.
Brenner BM, Cooper ME, de Zeeuw D, et al.: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001, 345:861–869.
Parving HH, Lehnert H, Brochner-Mortensen J, et al.: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001, 345:870–878.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993, 329:1456–1462.
Andersen S, Jacobsen P, Tarnow L, et al.: Time course of the antiproteinuric and antihypertensive effect of losartan in diabetic nephropathy. Nephrol Dial Transplant 2003, 18:293–297.
Ruilope LM, Segura J: Losartan and other angiotensin II antagonists for nephropathy in type 2 diabetes mellitus: a review of the clinical trial evidence. Clin Ther 2003, 25:3044–3064.
Jerums G, Allen TJ, Campbell DJ, et al.: Long-term comparison between perindopril and nifedipine in normotensive patients with type 1 diabetes and microalbuminuria. Am J Kidney Dis 2001, 37:890–899.
Chan JC, Ko GT, Leung DH, et al.: Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int 2000, 57:590–600.
Lacourciere Y, Belanger A, Godin C, et al.: Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int 2000, 58:762–769.
Ly J, Alexander M, Quaggin SE: A podocentric view of nephrology. Curr Opin Nephrol Hypertens 2004, 13:299–305. This review summarizes in detail the role of the podocyte slit pore diaphragm in controlling protein leakage.
Rantanen M, Palmen T, Patari A, et al.: Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 2002, 13:1586–1594.
Shih NY, Li J, Karpitskii V, et al.: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312–315.
Donoviel DB, Freed DD, Vogel H, et al.: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 2001, 21:4829–4836.
Cooper ME, Mundel P, Boner G: Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol 2002, 22:393–398. Detailed review about the role of nephrin with a focus on diabetic nephropathy, with a mechanistic assessment of treatments.
Hoshi S, Shu Y, Yoshida F, et al.: Podocyte injury promotes progressive nephropathy in Zucker diabetic fatty rats. Lab Invest 2002, 82:25–35.
Gross ML, El-Shakmak A, Szabo A, et al.: ACE-inhibitors but not endothelin receptor blockers prevent podocyte loss in early diabetic nephropathy. Diabetologia 2003, 46:856–868.
Benigni A, Gagliardini E, Tomasoni S, et al.: Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int 2004, 65:2193–2200. One of the first reports emphasizing the redistribution of nephrin in human diabetic nephropathy.
Toyoda M, Suzuki D, Umezono T, et al.: Expression of human nephrin mRNA in diabetic nephropathy. Nephrol Dial Transplant 2004, 19:380–385.
Koop K, Eikmans M, Baelde HJ, et al.: Expression of podocyteassociated molecules in acquired human kidney diseases. J Am Soc Nephrol 2003, 14:2063–2071.
Kelly DJ, Aaltonen P, Cox AJ, et al.: Expression of the slitdiaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant 2002, 17:1327–1332.
Davis BJ, Cao Z, de Gasparo M, et al.: Disparate effects of angiotensin II antagonists and calcium channel blockers on albuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens 2003, 21:209–216.
Bonnet F, Cooper ME, Kawachi H, et al.: Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia 2001, 44:874–847. One of the first reports showing that RAS blockade restores nephrin depletion in diabetic nephropathy.
Aaltonen P, Luimula P, Astrom E, et al.: Changes in the expression of nephrin gene and protein in experimental diabetic nephropathy. Lab Invest 2001, 81:1185–1190. Original contribution to the findings of nephrin gene and protein depletion and redistribution in experimental diabetic nephropathy.
Forbes JM, Bonnet F, Russo LM, et al.: Modulation of nephrin in the diabetic kidney: association with systemic hypertension and increasing albuminuria. J Hypertens 2002, 20:985–992.
Mifsud SA, Allen TJ, Bertram JF, et al.: Podocyte foot process broadening in experimental diabetic nephropathy: amelioration with renin-angiotensin blockade. Diabetologia 2001, 44:878–882.
Langham RG, Kelly DJ, Cox AJ, et al.: Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 2002, 45:1572–1576.
Lee EY, Shim MS, Kim MJ, et al.: Angiotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med 2004, 36:65–70. Original report showing that AT1 receptor antagonisms reduces podocyte VEGF expression in diabetic nephropathy.
Langham RG, Kelly DJ, Cox AJ, et al.: Angiotensin II-induced proteinuria and expression of the podocyte slit pore membrane protein, nephrin. Nephrol Dial Transplant 2004, 19:262–263.
Cao Z, Bonnet F, Candido R, et al.: Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol 2002, 13:1773–1787.
Forbes JM, Cooper ME, Oldfield MD, Thomas MC: Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol 2003, 14:S254-S258.
Vlassara H, Striker LJ, Teichberg S, et al.: Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A 1994, 91:11704–11708.
Forbes JM, Thallas V, Thomas MC, et al.: The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J 2003, 17:1762–1764.
Miyata T, van Ypersele de Strihou C, Ueda Y, et al.: Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol 2002, 13:2478–2487. Original report describing reduced AGE formation with RAS blockade in vitro.
Forbes JM, Cooper ME, Thallas V, et al.: Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes 2002, 51:3274–3282. First paper to demonstrate inhibition of AGE accumulation with ACE inhibition in vivo in a model of diabetic nephropathy.
Nangaku M, Miyata T, Sada T, et al.: Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J Am Soc Nephrol 2003, 14:1212–1222.
Nishikawa T, Edelstein D, Du XL, et al.: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404:787–790.
Cooper ME, Vranes D, Youssef S, et al.: Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 1999, 48:2229–2239. Original description of increased VEGF and its receptor expression in experimental diabetes emphasizing a pivotal role of VEGF for proteinuria.
Eremina V, Sood M, Haigh J, et al.: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003, 111:707–716.
de Vriese AS, Tilton RG, Elger M, et al.: Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 2001, 12:993–1000.
Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, et al.: Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 2002, 51:3090–3094. This paper shows that antagonism of VEGF with a neutralizing antibody ameliorated proteinuria and the progression of nephropathy in diabetes.
Flyvbjerg A: Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia 2000, 43:1205–1223.
Cha DR, Kim NH, Yoon JW, et al.: Role of vascular endothelial growth factor in diabetic nephropathy. Kidney Int Suppl 2000, 77:S104-S112.
Hovind P, Tarnow L, Oestergaard PB, Parving HH: Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl 2000, 75:S56-S61.
Zhang X, Lassila M, Cooper ME, Cao Z: Retinal expression of vascular endothelial growth factor is mediated by angiotensin type 1 and type 2 receptors. Hypertension 2004, 43:276–281.
Williams B, Baker AQ, Gallacher B, Lodwick D: Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension 1995, 25:913–917.
Koga K, Yamagishi S, Takeuchi M, et al.: CS-886, a new angiotensin II type 1 receptor antagonist, ameliorates glomerular anionic site loss and prevents progression of diabetic nephropathy in Otsuka Long-Evans Tokushima fatty rats. Mol Med 2002, 8:591–599.
Evans JL, Goldfine ID, Maddux BA, Grodsky GM: Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002, 23:599–622.
Wendt TM, Tanji N, Guo J, et al.: RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 2003, 162:1123–1137.
Ziyadeh FN, Hoffman BB, Han DC, et al.: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 2000, 97:8015–8020.
Chen S, Jim B, Ziyadeh FN: Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 2003, 23:532–543.
Schiffer M, Bitzer M, Roberts IS, et al.: Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 2001, 108:807–816.
Durvasula RV, Petermann AT, Hiromura K, et al.: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 2004, 65:30–39.
Mifsud S, Kelly DJ, Qi W, et al.: Intervention with tranilast attenuates renal pathology and albuminuria in advanced experimental diabetic nephropathy. Nephron Physiol 2003, 95:83–91.
Sharma K, Eltayeb BO, McGowan TA, et al.: Captopril-induced reduction of serum levels of transforming growth factorbeta1 correlates with long-term renoprotection in insulindependent diabetic patients. Am J Kidney Dis 1999, 34:818–823.
Shin GT, Kim SJ, Ma KA, et al.: ACE inhibitors attenuate expression of renal transforming growth factor-beta1 in humans. Am J Kidney Dis 2000, 36:894–902.
Cao Z, Cooper ME, Wu LL, et al.: Blockade of the reninangiotensin and endothelin systems on progressive renal injury. Hypertension 2000, 36:561–568.
Chaturvedi N, Schalkwijk CG, Abrahamian H, et al.: Circulating and urinary transforming growth factor beta1, Amadori albumin, and complications of type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care 2002, 25:2320–2327.
Riser BL, Denichilo M, Cortes P, et al.: Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol 2000, 11:25–38.
Roestenberg P, van Nieuwenhoven FA, Wieten L, et al.: Connective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathy. Diabetes Care 2004, 27:1164–1170.
Gilbert RE, Akdeniz A, Weitz S, et al.: Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care 2003, 26:2632–2636.
Riser BL, Cortes P, DeNichilo M, et al.: Urinary CCN2 (CTGF) as a possible predictor of diabetic nephropathy: preliminary report. Kidney Int 2003, 64:451–458.
Twigg SM, Cooper ME: The time to target connective tissue growth factor in diabetic complications is nigh. Diabetologia 2004, 47:965–968.
Sakharova OV, Taal MW, Brenner BM: Pathogenesis of diabetic nephropathy: focus on transforming growth factor-beta and connective tissue growth factor. Curr Opin Nephrol Hypertens 2001, 10:727–738.
Murphy M, Godson C, Cannon S, et al.: Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem 1999, 274:5830–5834.
Liu BC, Chen Q, Luo DD, et al.: Mechanisms of irbesartan in prevention of renal lesion in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2003, 24:67–73.
Lee HB, Yu MR, Yang Y, et al.: Reactive oxygen speciesregulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 2003, 14:S241-S245. This paper emphasizes the pivotal role of oxidative stress and related signaling pathways for the development and progression of diabetic nephropathy.
Guijarro C, Egido J: Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 2001, 59:415–424.
Mercurio F, Manning AM: NF-kappaB as a primary regulator of the stress response. Oncogene 1999, 18:6163–6171.
Mezzano S, Droguett A, Burgos ME, et al.: Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl 2003, S64–S70.
Esteban V, Ruperez M, Vita JR, et al.: Effect of simultaneous blockade of AT1 and AT2 receptors on the NFkappaB pathway and renal inflammatory response. Kidney Int Suppl 2003, 86:S33-S38. This report links both the angiotensin AT1 and AT2 receptors with overexpression of the transcription factor NF kappa B in diabetes.
Tuttle KR, Anderson PW: A novel potential therapy for diabetic nephropathy and vascular complications: protein kinase C beta inhibition. Am J Kidney Dis 2003, 42:456–465.
Hempel A, Maasch C, Heintze U, et al.: High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res 1997, 81:363–371.
Ishii H, Jirousek MR, Koya D, et al.: Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 1996, 272:728–731. Original report demonstrating the pivotal role of PKC beta for vascular dysfunction in diabetes.
Kelly DJ, Zhang Y, Hepper C, et al.: Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 2003, 52:512–518.
Koya D, Haneda M, Nakagawa H, et al.: Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J 2000, 14:439–447. This paper demonstrates the importance of PKC beta in a model of type 2 diabetic nephropathy.
Ikehara K, Tada H, Kuboki K, Inokuchi T: Role of protein kinase C-angiotensin II pathway for extracellular matrix production in cultured human mesangial cells exposed to high glucose levels. Diabetes Res Clin Pract 2003, 59:25–30.
Pfaff IL, Vallon V: Protein kinase C beta isoenzymes in diabetic kidneys and their relation to nephroprotective actions of the ACE inhibitor lisinopril. Kidney Blood Press Res 2002, 25:329–340.
Osicka TM, Yu Y, Panagiotopoulos S, et al.: Prevention of albuminuria by aminoguanidine or ramipril in streptozotocin-induced diabetic rats is associated with the normalization of glomerular protein kinase C. Diabetes 2000, 49:87–93.
Melhem MF, Craven PA, Liachenko J, DeRubertis FR: Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol 2002, 13:108–116.
Brands MW, Bell TD, Gibson B: Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension 2004, 43:57–63.
Jaimes EA, Galceran JM, Raij L: Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int 1998, 54:775–784.
de Cavanagh EM, Inserra F, Toblli J, et al.: Enalapril attenuates oxidative stress in diabetic rats. Hypertension 2001, 38:1130–1136.
Sugimoto K, Tsuruoka S, Fujimura A: Effect of enalapril on diabetic nephropathy in OLETF rats: the role of an anti-oxidative action in its protective properties. Clin Exp Pharmacol Physiol 2001, 28:826–830.
Russo LM, Bakris GL, Comper WD: Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis 2002, 39:899–919. This review suggests an alternative mechanism for proteinuria. It is hypothesized that impaired processing of albumin by tubular cells in various disease states, such as diabetic nephropathy, may play a major role in the pathogenesis of proteinuria in diabetes.
Russo LM, Brammar GC, Jerums G, et al.: The effect of ramipril on albumin excretion in diabetes and hypertension: the role of increased lysosomal activity and decreased transforming growth factor-beta expression. J Hypertens 2003, 21:419–428.
Clavant SP, Forbes JM, Thallas V, et al.: Reversible angiotensin II-mediated albuminuria in rat kidneys is dynamically associated with cytoskeletal organization. Nephron Physiol 2003, 93:51–60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lassila, M., Cooper, M.E. & Jandeleit-Dahm, K. Antiproteinuric effect of RAS blockade: New mechanisms. Current Science Inc 6, 383–392 (2004). https://doi.org/10.1007/s11906-004-0058-9
Issue Date:
DOI: https://doi.org/10.1007/s11906-004-0058-9