Abstract
Purpose of Review
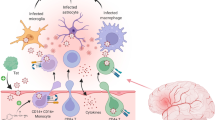
The purpose of this review is to summarize the current knowledge on the role of CD4+ T lymphocytes leading to HIV assault and persistence in the central nervous system (CNS) and the elimination of HIV-infected CNS resident cells by CD8+ T lymphocytes.
Recent Findings
HIV targets the CNS early in infection, and HIV-infected individuals suffer from mild forms of neurological impairments even under antiretroviral therapy (ART). CD4+ T cells and monocytes mediate HIV entry into the brain and constitute a source for HIV persistence and neuronal damage. HIV-specific CD8+ T cells are also massively recruited in the CNS in acute infection to control viral replication but cannot eliminate HIV-infected cells within the CNS.
Summary
This review summarizes the involvement of CD4+ T cells in seeding and maintaining HIV infection in the brain and describes the involvement of CD8+ T cells in HIV neuropathogenesis, playing a role still to be deciphered, either beneficial in eliminating HIV-infected cells or deleterious in releasing inflammatory cytokines.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Saloner R, Cysique LA. HIV-associated neurocognitive disorders: a global perspective. J Int Neuropsychol Soc. 2017;23(9–10):860–9. https://doi.org/10.1017/S1355617717001102.
Spudich S. HIV and neurocognitive dysfunction. Curr HIV/AIDS Rep. 2013;10(3):235–43. https://doi.org/10.1007/s11904-013-0171-y.
Farhadian S, Patel P, Spudich S. Neurological complications of HIV infection. Curr Infect Dis Rep. 2017;19(12):50. https://doi.org/10.1007/s11908-017-0606-5.
Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. https://doi.org/10.1016/j.bbi.2014.10.008.
• Do TC, Kerr SJ, Avihingsanon A, Suksawek S, Klungkang S, Channgam T, et al. HIV-associated cognitive performance and psychomotor impairment in a Thai cohort on long-term cART. J Virus Erad. 2018;4(1):41–7 This cross-sectional study evaluated the cognitive and psychomotor performance in well-supressed HIV-infected Thai individuals after long-term treatment compared with healthy individuals.
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–96. https://doi.org/10.1212/WNL.0b013e318200d727.
Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, et al. Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;69(1):29–35. https://doi.org/10.1097/QAI.0000000000000532.
• Anderson AM, Munoz-Moreno JA, McClernon DR, Ellis RJ, Cookson D, Clifford DB, et al. Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J Infect Dis. 2017;215(1):105–13. https://doi.org/10.1093/infdis/jiw505 In this study, the authors were able to detect a low level of HIV-1 RNA in the CSF of US HIV-infected participants under ART, using a sensitive detection method, and the discordent detection in the CSF compared with blood was associated with lower neurocognitive performance.
Chan P, Hellmuth J, Spudich S, Valcour V. Cognitive impairment and persistent CNS injury in treated HIV. Curr HIV/AIDS Rep. 2016;13(4):209–17. https://doi.org/10.1007/s11904-016-0319-7.
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72. https://doi.org/10.1097/01.aids.0000432467.54003.f7.
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol. 2011;17(1):3–16. https://doi.org/10.1007/s13365-010-0006-1.
Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2(1):12–21. https://doi.org/10.1002/acn3.136.
Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–9. https://doi.org/10.1097/01.aids.0000076308.76477.b8.
Kore I, Ananworanich J, Valcour V, Fletcher JL, Chalermchai T, Paul R, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70(4):393–9. https://doi.org/10.1097/QAI.0000000000000746.
D’Antoni ML, Byron MM, Chan P, Sailasuta N, Sacdalan C, Sithinamsuwan P, et al. Normalization of soluble CD163 after institution of antiretroviral therapy during acute HIV infection tracks with fewer neurological abnormalities. J Infect Dis. 2018;218:1453–63. https://doi.org/10.1093/infdis/jiy337.
Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–51. https://doi.org/10.1097/QAD.0b013e32834a40cd.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. https://doi.org/10.1038/nature14432.
Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6. https://doi.org/10.7554/eLife.29738.
Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun. 2013;34:11–6. https://doi.org/10.1016/j.bbi.2013.04.002.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. https://doi.org/10.1084/jem.20142290.
• Honeycutt JB, Liao B, Nixon CC, Cleary RA, Thayer WO, Birath SL, et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J Clin Invest. 2018;128:2862–76. https://doi.org/10.1172/JCI98968 This study used two mouse models to demonstrate that CD4 + T cells are sufficient to drive HIV infection in the brain.
Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neuro-Oncol. 2015;21(3):276–89. https://doi.org/10.1007/s13365-014-0287-x.
Smolders J, Remmerswaal EB, Schuurman KG, Melief J, van Eden CG, van Lier RA, et al. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol. 2013;126(4):525–35. https://doi.org/10.1007/s00401-013-1155-0.
Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular composition of cerebrospinal fluid in HIV-1 infected and uninfected subjects. PLoS One. 2013;8(6):e66188. https://doi.org/10.1371/journal.pone.0066188.
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. https://doi.org/10.1093/infdis/jis326.
Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep. 2015;12(1):16–24. https://doi.org/10.1007/s11904-014-0255-3.
Spudich SS. Immune activation in the central nervous system throughout the course of HIV infection. Curr Opin HIV AIDS. 2016;11(2):226–33. https://doi.org/10.1097/COH.0000000000000243.
Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad. 2015;1(2):67–71.
• Chan P, Patel P, Hellmuth J, Colby DJ, Kroon E, Sacdalan C, et al. Distribution of HIV RNA in CSF and blood is linked to CD4/CD8 ratio during acute HIV. J Infect Dis. 2018;218:937–45. https://doi.org/10.1093/infdis/jiy260 This study describes the detection of HIV RNA in CSF in most of the HIV individuals in acute infection and shows CSF HIV RNA peak at Fiebig IV subsequent to the peak in plasma.
Spudich SS, Ances BM. CROI 2017: neurologic complications of HIV infection. Top Antivir Med. 2017;25(2):69–76.
• Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. https://doi.org/10.1371/journal.ppat.1004720 This study compared CSF and blood viral populations in ART-naïve subjects within 2 years post-HIV infection and demonstrated four different mechanisms in HIV brain infection in which both T cell pleocyosis and local viral replication in the CNS occur in early infection.
Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, Miller AD, et al. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10(12):e1004533. https://doi.org/10.1371/journal.ppat.1004533.
• Hsu DC, Sunyakumthorn P, Wegner M, Schuetz A, Silsorn D, Estes JD, et al. Central nervous system inflammation and infection during early, non-accelerated simian-human immunodeficiency virus infection in rhesus macaques. J Virol. 2018;92:e00222–18. https://doi.org/10.1128/JVI.00222-18 This work addresses the very early events occuring in the brain after SIV infection and shows T cell infiltration without BBB damage.
Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12(2):85–96.
Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349(9053):692–5. https://doi.org/10.1016/S0140-6736(96)10178-1.
Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24(1):71–80. https://doi.org/10.1176/appi.neuropsych.11050109.
Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio. 2017;8(5). https://doi.org/10.1128/mBio.01280-17.
Veenstra M, Williams DW, Calderon TM, Anastos K, Morgello S, Berman JW. Frontline Science: CXCR7 mediates CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: a potential therapeutic target for NeuroAIDS. J Leukoc Biol. 2017;102(5):1173–85. https://doi.org/10.1189/jlb.3HI0517-167R.
Ndhlovu LC, D’Antoni ML, Ananworanich J, Byron MM, Chalermchai T, Sithinamsuwan P, et al. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naïve HIV-infected Thais. J Neuroimmunol. 2015;288:25–33. https://doi.org/10.1016/j.jneuroim.2015.08.020.
D'Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV following dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr. 2018;79:108–16. https://doi.org/10.1097/QAI.0000000000001752.
Dalvi P, Sun B, Tang N, Pulliam L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci Rep. 2017;7(1):9954. https://doi.org/10.1038/s41598-017-10449-0.
•• Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med. 2017;23(11):1271–6. https://doi.org/10.1038/nm.4411 This study quantifies the viral reservoirs in many tissues and demonstrates the persistence of HIV RNA+ cells in the brain after ART in SIV-infected monkeys.
Haupts M, Smektala K, Finkbeiner T, Simmet T, Gehlen W. Immunoreactive leukotriene C4 levels in CSF of MS patients. Acta Neurol Scand. 1992;85(5):365–7.
Bertin J, Jalaguier P, Barat C, Roy MA, Tremblay MJ. Exposure of human astrocytes to leukotriene C4 promotes a CX3CL1/fractalkine-mediated transmigration of HIV-1-infected CD4(+) T cells across an in vitro blood-brain barrier model. Virology. 2014;454-455:128–38. https://doi.org/10.1016/j.virol.2014.02.007.
Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93(1–2):172–81.
Beauparlant D, Rusert P, Magnus C, Kadelka C, Weber J, Uhr T, et al. Delineating CD4 dependency of HIV-1: adaptation to infect low level CD4 expressing target cells widens cellular tropism but severely impacts on envelope functionality. PLoS Pathog. 2017;13(3):e1006255. https://doi.org/10.1371/journal.ppat.1006255.
Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neuro-Oncol. 2015;21(3):227–34. https://doi.org/10.1007/s13365-014-0291-1.
Mallard J, Williams K. An SIV macaque model of SIV and HAND: the need for adjunctive therapies in HIV that target activated monocytes and macrophages. J Neuro-Oncol. 2018;24(2):213–9. https://doi.org/10.1007/s13365-018-0616-6.
Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. MBio. 2017;8(4). https://doi.org/10.1128/mBio.01186-17.
Barat C, Proust A, Deshiere A, Leboeuf M, Drouin J, Tremblay MJ. Astrocytes sustain long-term productive HIV-1 infection without establishment of reactivable viral latency. Glia. 2018;66:1363–81. https://doi.org/10.1002/glia.23310.
• Kessing CF, Spudich S, Valcour V, Cartwright P, Chalermchai T, Fletcher JL, et al. High number of activated CD8+ T cells targeting HIV antigens are present in cerebrospinal fluid in acute HIV infection. J Acquir Immune Defic Syndr. 2017;75(1):108–17. https://doi.org/10.1097/QAI.0000000000001301 This study characterizes the CD8 + T lymphocytes in the CSF of HIV-infected participants during acute infection and demonstrates the presence of functional HIV-specific CD8 + T cells compared with untreated chronic infection.
• Ganesh A, Lemongello D, Lee E, Peterson J, McLaughlin BE, Ferre AL, et al. Immune activation and HIV-specific CD8(+) T cells in cerebrospinal fluid of HIV controllers and noncontrollers. AIDS Res Hum Retrovir. 2016;32(8):791–800. https://doi.org/10.1089/AID.2015.0313 This study evidenced the surveillance of CNS by CD8 + T cells and demonstrated the presence of HIV-specific CD8 + T cells in CSF samples of controller and non-controller HIV-infected individuals at similar frequency.
Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, et al. CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity. 2016;45(3):656–68. https://doi.org/10.1016/j.immuni.2016.08.018.
• Richards MH, Narasipura SD, Seaton MS, Lutgen V, Al-Harthi L. Migration of CD8+ T cells into the central nervous system gives rise to highly potent anti-HIV CD4dimCD8bright T cells in a Wnt signaling-dependent manner. J Immunol. 2016;196(1):317–27. https://doi.org/10.4049/jimmunol.1501394 This study depicts a mice model reconstituted with human PBMCs and highlights a unique subset of potent anti-HIV CD8 + T cells expressing a low level of CD4 recruited in the brain.
Graham DR, Gama L, Queen SE, Li M, Brice AK, Kelly KM, et al. Initiation of HAART during acute simian immunodeficiency virus infection rapidly controls virus replication in the CNS by enhancing immune activity and preserving protective immune responses. J Neuro-Oncol. 2011;17(1):120–30. https://doi.org/10.1007/s13365-010-0005-2.
•• Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, et al. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017;9(377):eaag1809. https://doi.org/10.1126/scitranslmed.aag1809 This study characterizes HIV-specific CD8 + T cells in a unique cohort of HIV-infected individulas in acute infection and demonstrates the presence of HIV-specific CD8 + T cells in controling HIV replication in blood and limitating reservoir seeding.
•• Clayton KL, Collins DR, Lengieza J, Ghebremichael M, Dotiwala F, Lieberman J, et al. Resistance of HIV-infected macrophages to CD8(+) T lymphocyte-mediated killing drives activation of the immune system. Nat Immunol. 2018;19(5):475–86. https://doi.org/10.1038/s41590-018-0085-3 This study demonstrates a dichotomy in the capacity of CD8 + T cells to efficiently eliminate HIV-infected CD4 + T cells and macrophages and highlights an impaired macrophage killing leading to their activation and sustained inflammation.
• Yue FY, Cohen JC, Ho M, Rahman AK, Liu J, Mujib S, et al. HIV-specific granzyme B-secreting but not gamma interferon-secreting T cells are associated with reduced viral reservoirs in early HIV infection. J Virol. 2017;91(8). https://doi.org/10.1128/JVI.02233-16 This study examines INF-γ and granzyme B responses in PBMCs of HIV-infected individuals and demonstrates a granzyme B response from CD8 + T cells associated with a reduced viral reservoir.
Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, et al. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One. 2015;10(2):e0116526. https://doi.org/10.1371/journal.pone.0116526.
•• Grauer OM, Reichelt D, Gruneberg U, Lohmann H, Schneider-Hohendorf T, Schulte-Mecklenbeck A, et al. Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol. 2015;2(9):906–19. https://doi.org/10.1002/acn3.227 This study correlated increased frequency of activation markers on T lymphocytes in blood and in CSF with worse neurocognitive performance. A lower CD4/CD8 ratio was also associated with neurocognitive impairment.
Ratto-Kim S, Schuetz A, Sithinamsuwan P, Barber J, Hutchings N, Lerdlum S, et al. Characterization of cellular immune responses in Thai individuals with and without HIV-associated neurocognitive disorders. AIDS Res Hum Retrovir. 2018;34:685–9. https://doi.org/10.1089/AID.2017.0237.
Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120(17):3466–77. https://doi.org/10.1182/blood-2012-04-422550.
Uzasci L, Nath A, Cotter R. Oxidative stress and the HIV-infected brain proteome. J NeuroImmune Pharmacol. 2013;8(5):1167–80. https://doi.org/10.1007/s11481-013-9444-x.
Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24(3):284–90. https://doi.org/10.1097/WCO.0b013e328346be57.
Funding
This work was supported by the U.S. National Institute of Mental Health R01MH106466 and by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Central Nervous System and Cognition
Rights and permissions
About this article
Cite this article
Subra, C., Trautmann, L. Role of T Lymphocytes in HIV Neuropathogenesis. Curr HIV/AIDS Rep 16, 236–243 (2019). https://doi.org/10.1007/s11904-019-00445-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-019-00445-6