Abstract
Purpose of the Review
We aimed to provide an overview of telemedical monitoring and its impact on outcomes among heart failure (HF) patients.
Recent Findings
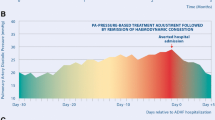
Most HF readmissions may be prevented if clinical parameters are strictly controlled via telemedical monitoring. Predictive algorithms for patients with cardiovascular implantable electronic devices (e.g., Triage-HF Plus by Medtronic or HeartLogic by Boston Scientific) were developed to identify patients at significantly increased risk of HF events. However, randomized control trial-based data are heterogeneous regarding the advantages of telemedical monitoring in HF patients. The likelihood of adverse clinical outcomes increases when pulmonary artery pressure (PAP) rises, usually days to weeks before clinical manifestations of HF. A wireless monitoring system (CardioMEMS™) detecting changes in PAP was proposed for HF patients. CardioMEMS™ transmits data to the healthcare provider and allows to institute timely intensification of HF therapies. CardioMEMS™-guided pharmacotherapy reduced a risk of HF-related hospitalization (hazard ratio [HR]: 0.72; 95% confidence interval (CI) 0.60–0–0.85; p < 0.01).
Summary
Relevant developments and innovations of telemedical care may improve clinical outcomes among HF patients. The use of CardioMEMS™ was found to be safe and cost-effective by reducing the rates of HF hospitalizations.
Similar content being viewed by others
Introduction
Heart failure (HF) is one of the most common cardiovascular diseases, with an estimated prevalence of 1–2% of the adults in the developed countries; and rising to > 10% in patients aged > 70 years [1,2,3]. HF accounts for over 1 million hospitalizations in the US and Europe annually [4]. Given that, an increasing number of patients with HF and a high rate of mortality and morbidity have serious public health and economic consequences.
A range of treatments has been shown to improve the survival and quality of life in HF patients, including pharmacotherapy, cardiovascular implantable electronic devices (CIEDs), implantable left ventricular assist device (LVAD), and heart transplant [5]. Optimal pharmacotherapy consists of beta-blockers, angiotensin-converting enzyme inhibitors, and mineralocorticoid receptor antagonists (if left ventricular ejection fraction [LVEF] ≤ 35%) [5]. Recently, new drugs such as the angiotensin receptor neprilysin inhibitor (sacubitril/valsartan) and inhibitors of sodium–glucose cotransporter 2 (dapagliflozin) have shown better outcomes and survival benefits in HF patients when added to standard pharmacotherapy [6, 7].
Following optimal pharmacotherapy, CIEDs have become the cornerstone of management for patients with brady- or tachyarrhythmias and HF [5, 8,9,10]. CIEDs include complex stimulation systems–pacemakers (PM), implantable cardioverter defibrillators (ICD), and cardiac resynchronization therapy (CRT). It is well-established that rhythm management devices improve the life expectancy and quality of life of HF patients [11].
Despite all the treatment options, the prognosis of HF patients is poor; and patients are still admitted to the hospital with HF worsening or arrhythmia. Acute exacerbations of HF often require prolonged in-hospital treatments and contribute to adverse prognosis [12, 13]. However, the majority of HF readmissions are due to fluid overload, and the process of decompensation may be prevented if clinical parameters are strictly controlled via telemedical monitoring [14, 15]. The likelihood of adverse clinical outcomes increases when pulmonary artery pressure (PAP) rises, usually days to weeks before clinical manifestations of HF [16, 17]. A wireless monitoring system (CardioMEMS™) detecting changes in PAP has been proposed for HF patients. CardioMEMS™ transmits data to the healthcare provider and allows to institute timely intensification of HF therapies [18,19,20]. In this narrative review, we aimed to provide an overview of telemedical monitoring and its impact on outcomes among HF patients; focusing on the advances of the CardioMEMS™ system.
Search Strategy
A comprehensive literature search was performed, and the relevant studies and systematic reviews were identified. The following search terms were included: heart failure, telemedical monitoring, remote monitoring, CardioMEMS™ system, cardiac implantable electronic devices, pacemaker, implantable cardioverter defibrillator, cardiac resynchronization therapy, and pulmonary artery pressure monitoring. The selected articles and guideline documents were reviewed for inclusion.
Home Monitoring in HF Patients
The “first step” of home monitoring began with daily heart rate and blood pressure measurements performed by patients at home. Poor patient compliance limit the impact on overall care. When combined with structured control, e.g., telephone calls, the effects were better. A meta-analysis of 20 randomized control trials (RCTs) and 12 cohort studies assessed the impact of remote patient monitoring (via regularly scheduled structured telephone contact between patients and health care providers or electronic transfer of data) on HF patients’ outcomes compared to usual care [21]. Telemedical care was associated with a significantly lower number of deaths (RCTs: relative risk [RR]: 0.83; 95% CI: 0.73–0.95; cohort studies: RR: 0.53; 95% CI: 0.29–0.96) and hospitalizations (RCTs: RR: 0.93; 95% CI: 0.87–0.99; cohort studies: RR: 0.52; 95% CI: 0.28–0.96) [21]. A different meta-analysis compared structured telephone support (n = 5613 participants) and telemonitoring (n = 2710 participants) versus standard practice for HF patients to quantify the effects of these interventions [22]. Telemonitoring reduced all-cause mortality (RR: 0.66; 95% CI: 0.54–0.81), while structured telephone had a non-significant positive effect (RR: 0.88, 95% CI: 0.76–1.01) as compared to the usual care. Both telephone support (RR: 0.77, 95% CI: 0.68–0.87) and telemonitoring (RR: 0.79, 95% CI 0.67–0.94) reduced HF-related hospitalizations [22]. However, most studies, when analyzed individually, failed to show significant reductions in hospitalization or mortality.
Remote Monitoring in Patients with CIEDs
Patients with CIEDs have routine in-person appointments every 6–12 months, and unscheduled clinic visits may be required in any case of device malfunction or worsening of health [23]. However, the “conventional” monitoring of the patients with CIEDs is inefficient and outdated [24, 25]. According to expert consensuses, telemedical monitoring should be made available for all patients with CIEDs, particularly for those with HF and cardiac arrhythmias [23, 26]. Furthermore, digital healthcare models and telemedical controls involve patients taking an active role in their clinical care. Such a personalized approach is the future of modern medicine and cardiology [27].
A telemedical system transfers the recorded data from the patient’s device to a database; new systems can even transmit the data via the patient’s smartphone. The data are available to the healthcare team on an ongoing basis. Thus, telemonitoring allows assessing the relevant technical parameters of the device (battery status, electrode function, and system compatibility) and provides other key clinical information (stimulation percentage, arrhythmic episodes, or current intracardiac electrogram) [23, 26]. In addition, novel telemetric strategies may provide data on the current clinical status or device alarms, including device-related malfunctions, arrhythmias, heart and respiratory rate statistics, heart sounds, and intrathoracic impedance [28,29,30,31].
Individual parameters have a poor predictive value of HF decompensation [32]. However, when combined, allow the implementation of predictive algorithms (e.g., Triage-HF Plus by Medtronic or HeartLogic by Boston Scientific) to identify patients at significantly increased risk of HF events [33, 34]. Given that telemedical monitoring may detect early signs and symptoms of HF decompensation, and the healthcare team may timely modify the pharmacotherapy and prevent HF hospitalization or incidence of inappropriate ICD shocks. Hence, this could be considered an ongoing “triage” of patients requiring an urgent intervention [29]. A recent EHRA survey [35] showed that the early detection of AF in PM patients, lead failure in ICD patients, and HF-worsening in CRT patients were essential advantages of telemedical monitoring.
However, RCT-based data are heterogeneous regarding the advantages of telemedical monitoring in HF patients (Table 1) [32, 36,37,38,39,40,41,42,43,44,45]. In the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial, 1339 patients with ICD were randomized to telemedical care with daily transmissions or conventional care (office visits only) [44]. Telemedical monitoring reduced the number of in-hospital visits and the time to detect arrhythmic events (1 vs. 36 days, respectively, p < 0.01) [44]. Further studies confirmed that telemedical monitoring reduced the time from the event onset (arrhythmias, disease progression, and device malfunctions) to a clinical decision (4.6 vs. 22 days; respectively, p < 0.01) [43]. However, only two studies showed improved clinical outcomes for HF patients by using telemedical care [36, 40]. Conversely, Böhm et al. reported that fluid status alerts did not improve outcomes (the composite of all-cause death and cardiovascular hospitalization) among ICD patients with advanced HF [45]. Furthermore, the remote monitoring: an evaluation of implantable devices for the management of Heart Failure patients (REM-HF) study showed that telemedical care using weekly downloads and a formalized follow-up did not reduce all-cause mortality and HF-related hospitalizations [39].
CardioMEMS™ Technology
The CardioMEMS™ (CardioMEMS HF System, Abbott, Sylmar, CA) is an implantable wireless sensor placed in the left lower lobe pulmonary artery (through a catheter-delivery system), capable of remotely measuring PAP [18]. It is the only invasive HF remote monitoring sensor with Food and Drug Administration (FDA) approval and European Conformity (CE) mark [19]. Patients take daily home measurements with an external electronics system and transmit the PAP data wirelessly for clinician review [18, 19]. The recent studies showed that the CardioMEMS™ reduced HF hospitalizations and improved quality of life (Table 2) [46,47,48,49,50,51].
The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA functional class III heart failure patients (CHAMPION) trial was an RCT conducted in 64 centers in the USA [46]. The inclusion criterion was the presence of HF in NYHA class III (irrespective of LVEF), and patients were randomized to PAP-guided therapy (n = 270) or standard care (n = 280). This trial found that hemodynamic-guided pharmacotherapy reduced HF hospitalization risk in outpatients (rate of HF-related hospitalizations at 6 months: 0.32 vs. 0.44; HR: 0.72; 95% CI 0.60–0.85, p < 0.01) [46]. A sub-analysis of the CHAMPION trial among patients with implanted CRT [52] showed that PAP‐guided adjustment of medical therapies decreased the burden of HF symptoms and hospitalizations (beyond the effect of CRT) by 30% compared with standard therapy (0.46 events/patient‐year vs. 0.68 events/patient‐year; HR: 0.70; 95% CI: 0.51–0.96, p = 0.03). Treatment patients had more medication titrations (847 vs. 346 in control, p < 0.01), reduction in mean PAP (− 413.2 ± 123.5 vs. 60.1 ± 88.0 in control, p < 0.01), and improvement in quality of life (Minnesota Living with Heart Failure Questionnaire decreased − 13.5 ± 23 vs. − 4.9 ± 24.8 in control, p < 0.01) [52]. Furthermore, a subanalysis of CHAMPION trial among patients with HF and reduced LVEF showed that patients receiving optimal PAP-guided therapy had lower HF hospitalizations (HR: 0.57; 95% CI: 0.45 − 0.74, p < 0.01) and 57% lower mortality (HR: 0.43; 95% CI: 0.24 − 0.76, p < 0.01) compared with the control group [53].
The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) provided the first European experience with PAP-guided therapy [49]. It was found that the use of CardioMEMS™ is safe and feasible, with 98.3% of patients remaining free from device- or system-related complications. Physician-directed management based on remotely obtained PAP values were associated with a 62% decrease in HF hospitalizations (at 12 months post- vs. pre-implant, HF hospitalizations: 0.60 vs. 1.55 events/patient-year; HR: 0.38; 95% CI: 0.31–0.48, p < 0.01), and marked increase in Kansas City Cardiomyopathy Questionnaire (overall summary score 47.0 ± 24.0 to 60.5 ± 24.3, p < 0.01) [49].
These consistent results support a role for remote PAP monitoring in guiding HF management in outpatients [46,47,48,49,50,51]. However, in ‘real-life’ practice, this strategy requires commercially available devices and adequate monitoring frequency (gained by patients and healthcare team) with appropriate training to translate the data into appropriate HF treatment modifications. For example, patients should consistently perform their daily PAP measurements early in the morning to improve the CardioMEMS interpretation [54]. It may also indicate actual changes in the patient’s status, rather than “time-of-day-dependent” variations [54]. The patients’ awareness of being monitored is an integral part of this strategy; treatment optimization relies on individual willingness and ability to collaborate [55]. This approach also requires patients’ self-motivation to undergo remote HF management, and timely PAP reassessment must inform caregivers and patients whether their intervention was effective. Notwithstanding these challenges, daily PAP measurements by patients, weekly trend review by healthcare providers, targeted medical interventions, and follow-up of treatment effects are needed. Moreover, each element of this PAP-based “package of care” is essential to the final “success” of hemodynamic-guided HF management.
In the USA, from May 28, 2014 (FDA premarket approval) to May 28, 2017, there were approximately 5500 CardioMEMS™ implants [56]. During this interval, 177 adverse events (e.g., pulmonary artery injury/hemoptysis, sensor failure/malfunction/migration, access site-related bleeding/infection, and pulmonary embolism/device thrombosis)–including 22 procedure-related deaths (0.4%) were reported [56]. Hence, candidate selection, operator training, and technological refinement may improve device safety and durability.
Challenges and Future Directions
Telemedical monitoring is not the treatment per se, but it is a tool for better-addressing healthcare requirements, allowing timely medical response to device alerts [40, 57]. The benefits of telemedical monitoring may vary–based on the healthcare reaction to the transmitted data and the level of patient adherence [58]. Therefore, further developments should be focused on improving the feasibility and efficiency of telemonitoring, e.g., artificial intelligence to “triage” patients or integration of extra features to monitor potential comorbidities (blood pressure and sugar levels) [24]. Likewise, artificial intelligence may support diagnostic and treatment decisions, including predicting arrhythmias or other cardiovascular diseases [59, 60].
Further studies may identify the novel functions with a positive impact on clinical outcomes in HF patients (e.g., new sensors capable of measuring left atrial pressures), and evaluation of “real world” data will help define its role in HF management [20, 24, 25, 61, 62]. For example, the HEMOdynamic guidance with CardioMEMS in patients with a left Ventricular Assist Device (HEMO-VAD) study was the first prospective pilot study assessing the safety and feasibility of the CardioMEMS™ for optimization of LVAD therapy [63]; and The Hemodynamic-GUIDEd Management of Heart Failure (GUIDE-HF) is ongoing RCT to demonstrate the effectiveness of the CardioMEMS™ in an expanded patient population (HF patients regardless of ejection fraction in NYHA class II–IV) [64]. Furthermore, the CardioMEMS Post-Market Multinational Clinical Study (COAST) will investigate the generalizability of remote PAP-guided management in several national settings (85 sites across Europe and Australia) [65].
However, telemedical monitoring is still underused in clinical practice. Challenges to its implementation are the lack of reimbursement, the adherence to therapeutic protocols by physicians and patients, and the need for significant changes in hospitals’ workflows or data overload [57, 66,67,68]. Telemedical monitoring is particularly relevant to prevent hospitalization and reduce the requirement for “face to face” follow-up, for example, to keep social distancing during the current COVID-19 pandemic [69, 70]. Indeed, the advancement of digital health strategy, including smartphones, wearables, and telemedical monitoring, maybe an unexpected outcome of the COVID-19 pandemic [71, 72].
Conclusion
Relevant developments and innovations of telemedical monitoring may improve clinical outcomes among HF patients. The CardioMEMS™ was found to be safe and cost-effective by reducing the rates of heart failure hospitalizations. Telemedical care responds to the unmet need of HF hospitalization and death prevention and should therefore be recommended as part of multidisciplinary management of HF patients.
References
Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11.
Groenewegen A, Rutten FH, Mosterd A, et al. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–56.
Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England) 2018; 392: 1789–1858.
Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–33.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200m.
McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Brignole M, Auricchio A, Baron-Esquivias G, et al. 213 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–329.
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–391.
Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the europe. Eur Heart J. 2015;36:2793–867.
Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the heart rhythm society (HRS) and. EP Eur. 2008;10:707–25.
Chun S, Tu JV, Wijeysundera HC, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–21.
Bergethon KE, Ju C, DeVore AD, et al. 2016 Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the get with the guidelines-heart failure registry. Circ Heart Fail; 9. Epub ahead of print June. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002594.
Joseph SM, Cedars AM, Ewald GA, et al. Acute decompensated heart failure: contemporary medical management. Texas Hear Inst J. 2009;36:510–20.
Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Prim. 2020;6:16.
Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3:580–7.
Zile MR, Bennett TD, El Hajj S, et al. Intracardiac pressures measured using an implantable hemodynamic monitor. Circ Hear Fail. 2017;10:e003594.
Abraham J, McCann PJ, Guglin ME, et al. Management of the patient with heart failure and an implantable pulmonary artery hemodynamic sensor. Curr Cardiovasc Risk Rep. 2020;14:12.
Brugts JJ, Radhoe SP, Aydin D, et al. 2021 Clinical update of the latest evidence for CardioMEMS pulmonary artery pressure monitoring in patients with chronic heart failure: a promising system for remote heart failure care. Sensors (Basel); 21. Epub ahead of print March. https://doi.org/10.3390/s21072335.
Mangi MA, Nesheiwat Z, Kahloon R, et al. CardioMEMSTM System in the daily management of heart failure: review of current data and technique of implantation. Expert Rev Med Devices. 2020;17:637–48.
Klersy C, De Silvestri A, Gabutti G, et al. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54:1683–94.
Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane database Syst Rev. 2010;4(8):CD007228.
Yee R, Verma A, Beardsall M, et al. Canadian cardiovascular society/Canadian heart rhythm society joint position statement on the use of remote monitoring for cardiovascular implantable electronic device follow-up. Can J Cardiol. 2013;29:644–51.
Kotalczyk A, Kalarus Z, Wright DJ, et al. Cardiac electronic devices: future directions and challenges. Medical Devices: Evidence and Research. 2020;13:325–38.
Imberti JF, Tosetti A, Mei DA, et al. Remote monitoring and telemedicine in heart failure : implementation and benefits.
Slotwiner D, Varma N, Akar JG, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Hear Rhythm. 2015;12:e69–100.
Frederix I, Caiani EG, Dendale P, et al. ESC e-cardiology working group position paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol. 2019;26:1166–77.
Ontario HQ. Remote monitoring of implantable cardioverter-defibrillators, cardiac resynchronization therapy and permanent pacemakers: a health technology assessment. Ont Health Technol Assess Ser. 2018;18:1–199.
Liberska A, Kowalski O, Mazurek M, et al. Day by day telemetric care of patients treated with cardiac resynchronisation therapy: first Polish experience. Kardiol Pol. 2016;74:741–8.
Boriani G, Imberti JF, Vitolo M. Atrial fibrillation and remote monitoring through cardiac implantable electronic devices in heart failure patients. Eur J Heart Fail. 2020;22(3):554–6.
Baginski BN, Byrne KA, Vaz DG, et al. Development and implementation of a remote patient monitoring program for heart failure: a single-centre experience. ESC Hear Fail. 2021;8:1349–58.
van Veldhuisen DJ, Braunschweig F, Conraads V, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–26.
Gardner RS, Singh JP, Stancak B, et al. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events. Circ Hear Fail. 2018;11:e004669.
Ahmed FZ, Taylor JK, Green C, et al. Triage-HF Plus: a novel device-based remote monitoring pathway to identify worsening heart failure. ESC Hear Fail. 2020;7:108–17.
Mairesse GH, Braunschweig F, Klersy K, et al. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics committee of the European Heart Rhythm Association. EP Eur. 2015;17:814–8.
Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–90.
Sardu C, Santamaria M, Rizzo MR, et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J Clin Pract. 2016;70:569–76.
Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the more-care multicentre randomized controlled trial. Eur J Heart Fail. 2017;19:416–25.
Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–60.
Tajstra M, Sokal A, Gadula-Gacek E, et al. Remote supervision to decrease hospitalization rate (RESULT) study in patients with implanted cardioverter-defibrillator. EP Eur. 2020;22:769–76.
Landolina M, Perego GB, Lunati M, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125:2985–92.
Heidbuchel H, Hindricks G, Broadhurst P, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. 2015;36:158–69.
Crossley GH, Boyle A, Vitense H, et al. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–9.
Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T safely reduces routine office device follow-up (TRUST) trial. Circulation. 2010;122:325–32.
Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37:3154–63.
Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66.
Shavelle DM, Desai AS, Abraham WT, et al. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail. 2020;13:e006863.
Assaad M, Sarsam S, Naqvi A, et al. CardioMems® device implantation reduces repeat hospitalizations in heart failure patients: a single center experience. JRSM Cardiovasc Dis. 2019;8:2048004019833290.
Angermann CE, Assmus B, Anker SD, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European monitoring study for heart failure (MEMS-HF). Eur J Heart Fail. 2020;22:1891–901.
Abraham J, Bharmi R, Jonsson O, et al. Association of ambulatory hemodynamic monitoring of heart failure with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiol. 2019;4:556–63.
Jermyn R, Alam A, Kvasic J, et al. Hemodynamic-guided heart-failure management using a wireless implantable sensor: infrastructure, methods, and results in a community heart failure disease-management program. Clin Cardiol. 2017;40:170–6.
Varma N, Bourge RC, Stevenson LW, et al. Remote hemodynamic-guided therapy of patients with recurrent heart failure following cardiac resynchronization therapy. J Am Heart Assoc. 2021;10:e017619–e017619.
Givertz MM, Stevenson LW, Costanzo MR, et al. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017;70:1875–86.
Crnko S, Brugts JJ, Veenis JF, et al. Morning pulmonary artery pressure measurements by CardioMEMS are most stable and recommended for pressure trends monitoring. Netherlands Hear J Mon J Netherlands Soc Cardiol Netherlands Hear Found. 2021;29(7–8):409–14. https://doi.org/10.1007/s12471-021-01590-7.
Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the heart failure association of the European society of cardiology. Eur J Heart Fail. 2021;23:157–74.
Vaduganathan M, DeFilippis EM, Fonarow GC, et al. Postmarketing adverse events related to the CardioMEMS HF system. JAMA Cardiol. 2017;2:1277–9.
Braunschweig F, Anker SD, Proff J, et al. Remote monitoring of implantable cardioverter-defibrillators and resynchronization devices to improve patient outcomes: dead end or way ahead? EP Eur. 2019;21:846–55.
Varma N, Piccini JP, Snell J, et al. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65:2601–10.
Pevnick JM, Birkeland K, Zimmer R, et al. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc Med. 2018;28:144–50.
Silva-Cardoso J, Juanatey JRG, Comin-Colet J, et al. The future of telemedicine in the management of heart failure patients. Card Fail Rev. 2021;7:e11.
Goette A, Auricchio A, Boriani G, et al. EHRA white paper: knowledge gaps in arrhythmia management - status 2019. Europace. 2019;21:993–4.
Radhoe SP, Veenis JF, Brugts JJ. 2021 Invasive devices and sensors for remote care of heart failure patients. Sensors (Basel); 21. Epub ahead of print March. https://doi.org/10.3390/s21062014.
Veenis JF, Radhoe SP, van Mieghem NM, et al. Remote hemodynamic guidance before and after left ventricular assist device implantation: short-term results from the HEMO-VAD pilot study. Future Cardiol. 2021;17(5):885–98. https://doi.org/10.2217/fca-2020-0182.
Lindenfeld J, Abraham WT, Maisel A, et al. Hemodynamic-GUIDEd management of heart failure (GUIDE-HF). Am Heart J. 2019;214:18–27.
Cowie MR, de Groote P, McKenzie S, et al. Rationale and design of the CardioMEMS post-market multinational clinical study: COAST. ESC Hear Fail. 2020;7:865–72.
Zanotto G, Melissano D, Baccillieri S, et al. Intrahospital organizational model of remote monitoring data sharing, for a global management of patients with cardiac implantable electronic devices: a document of the Italian association of arrhythmology and cardiac pacing. J Cardiovasc Med (Hagerstown). 2020;21:171–81.
Palmisano P, Melissano D, Zanotto G, et al. Change in the use of remote monitoring of cardiac implantable electronic devices in Italian clinical practice over a 5-year period: results of two surveys promoted by the AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J Cardiovasc Med (Hagerstown). 2020;21:305–14.
Maines M, Tomasi G, Moggio P, et al. Implementation of remote follow-up of cardiac implantable electronic devices in clinical practice: organizational implications and resource consumption. J Cardiovasc Med (Hagerstown). 2020;21:648–53.
McIlvennan CK, Allen LA, Devore AD, et al. Changes in care delivery for patients with heart failure during the COVID-19 pandemic: results of a multicenter survey. J Card Fail. 2020;26:635–6.
Oseran AS, Afari ME, Barrett CD, et al. Beyond the stethoscope: managing ambulatory heart failure during the COVID-19 pandemic. ESC Hear Fail. 2021;8:999–1006.
Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the heart rhythm society COVID-19 task force; electrophysiology section of the American College of Cardiology; and the electrocardiography and arrhythmias committee of the council on. Hear Rhythm. 2020;17:e233–41.
Mattioli AV, Cossarizza A, Boriani G. COVID-19 pandemic: usefulness of telemedicine in management of arrhythmias in elderly people. J Geriatr Cardiol. 2020;17:593–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
DJW: research grants and consultancy fees from Boston Scientific and Medtronic; GYHL: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are received personally.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Digital Medicine in Heart Failure
Rights and permissions
About this article
Cite this article
Kotalczyk, A., Imberti, J.F., Lip, G.Y.H. et al. Telemedical Monitoring Based on Implantable Devices—the Evolution Beyond the CardioMEMS™ Technology. Curr Heart Fail Rep 19, 7–14 (2022). https://doi.org/10.1007/s11897-021-00537-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-021-00537-8