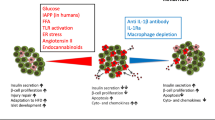
Abstract
Type 1 diabetes (T1D) is a disease that in most individuals results from autoimmune attack of a single tissue type, the pancreatic islet. A fundamental, unanswered question in T1D pathogenesis is how the islet tissue environment influences immune regulation. This crosstalk is likely to be communicated through the extracellular matrix (ECM). Here, we review what is known about the ECM in insulitis and examine how the tissue environment is synchronized with immune regulation. In particular, we focus on the role of hyaluronan (HA) and its interactions with Foxp3+ regulatory T-cells (Treg). We propose that HA is a “keystone molecule” in the inflammatory milieu and that HA, together with its associated binding proteins and receptors, is an appropriate point of entry for investigations into how ECM influences immune regulation in the islet.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Andre I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A. 1996;3:2260–3.
Capitan JA, Cuesta JA. Species assembly in model ecosystems, I: analysis of the population model and the invasion dynamics. J Theor Biol. 2011;269:330–43.
Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61.
Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–8.
Edelstam GA, Laurent UB, Lundkvist OE, Fraser JR, Laurent TC. Concentration and turnover of intraperitoneal hyaluronan during inflammation. Inflammation. 1992;16:459–69.
Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–404.
Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:12634–9.
Stern R, Jedrzejas MJ. Hyaluronidases. their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39.
Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715.
•• Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. This review is a comprehensive and accessible resource for anyone interested in hyaluronan studies.
Hull RL, Johnson PY, Braun KR, Day AJ, Wight TN. Hyaluronan and hyaluronan binding proteins are normal components of mouse pancreatic islets and are differentially expressed by islet endocrine cell types. J Histochem Cytochem. 2012;in press.
Powell JD, Horton MR. Threat matrix. Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–18.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:a006049.
Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111.
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9.
Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–35.
Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–81.
del Fresco C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol. 2005;174:3032–40.
Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–8.
Bollyky PL, Bice JB, Sweet IR, Falk BA, Gebe JA, Clark AE, et al. The toll-like receptor signaling molecule Myd88 contributes to pancreatic β-cell homeostasis in response to injury. PLoS One. 2009;4:e5063.
Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin Invest Med. 2008;31:E106–16.
Wei L, Xiong H, Li B, Gong Z, Li J, Cai H, et al. Change of HA molecular size and boundary lubrication in synovial fluid of patients with temporomandibular disorders. J Oral Rehabil. 2010;37:271–7.
• Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, et al. Role of hyaluronan and hyaluronan‐binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–11. These studies are a good introduction into how HA size and HA binding partners can influence inflammation.
Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189–206.
Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22.
Termeer C, Sleeman JP, Simon JC. Hyaluronan–magic glue for the regulation of the immune response? Trends Immunol. 2003;24:112–4.
Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;1:221–64.
Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta. 2011;1812:1170–81.
• Nagy N, Freudenberger T, Melchior-Becker A, Rock K, Ter BM, Jastrow H, et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122:2313–22. This work is notable in part because it is one of the first to examine the impact of loss of HA synthesis in an inflammatory disease.
Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, et al. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-β. J Immunol. 2009;183:2232–41.
Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, et al. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–75.
van der Windt GJ, Hoogendijk AJ, de Vos AF, Kerver ME, Florquin S, van der Poll T. The role of CD44 in the acute and resolution phase of the host response during pneumococcal pneumonia. Lab Invest. 2011;91:588–97.
Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–33.
Kimura K, Nagaki M, Kakimi K, Saio M, Saeki T, Okuda Y, et al. Critical role of CD44 in hepatotoxin-mediated liver injury. J Hepatol. 2008;48:952–61.
Chen D, McKallip RJ, Zeytun A, Do Y, Lombard C, Robertson JL, et al. CD44-deficient mice exhibit enhanced hepatitis after concanavalin A injection: evidence for involvement of CD44 in activation-induced cell death. J Immunol. 2001;166:5889–97.
Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–33.
Mylona E, Jones KA, Mills ST, Pavlath GK. CD44 regulates myoblast migration and differentiation. J Cell Physiol. 2006;209:314–21.
• Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, et al. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J Biol Chem. 2010;285:30170–80. This is an excellent study of the mechanisms underlying the binding interactions between CD44 and LMW-HA and HMW-HA.
Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4 + CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–72.
Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–43.
Peng ST, Su CH, Kuo CC, Shaw CF, Wang HS. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int J Oncol. 2007;31:1119–26.
Oertli B, Fan X, Wuthrich RP. Characterization of CD44-mediated hyaluronan binding by renal tubular epithelial cells. Nephrol Dial Transplant. 1998;13:271–8.
Fujii Y, Fujii K, Nakano K, Tanaka Y. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45–50.
Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–11.
Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4 + Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–903.
Long SA, Rieck M, Tatum M, Bollyky PL, Wu RP, Muller I, Ho JC, Shilling HG, Buckner JH. Low-dose antigen promotes induction of FOXP3 in human CD4+ T Cells. J Immunol. 2011.
Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9.
Bollyky PL, Evanko SP, Wu RP, Potter-Perigo S, Long SA, Kinsella B, et al. Th1 cytokines promote T-cell binding to antigen-presenting cells via enhanced hyaluronan production and accumulation at the immune synapse. Cell Mol Immunol. 2010;7:211–20.
Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Pure E. Fibroblast migration is mediated by CD44-dependent TGF β activation. J Cell Sci. 2008;121:1393–402.
David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, et al. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274–87.
Kawana H, Karaki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, et al. CD44 suppresses TLR-mediated inflammation. J Immunol. 2008;180:4235–45.
Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–43.
Mio K, Stern R. Inhibitors of the hyaluronidases. Matrix Biol. 2002;21:31–7.
Kvezereli M, Michie SA, Yu T, Creusot RJ, Fontaine MJ. TSG-6 protein expression in the pancreatic islets of NOD mice. J Mol Histol. 2008;39:585–93.
Bardos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am J Pathol. 2001;159:1711–21.
Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–23.
Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100.
Sainio A, Jokela T, Tammi MI, Jarvelainen H. Hyperglycemic conditions modulate connective tissue reorganization by human vascular smooth muscle cells through stimulation of hyaluronan synthesis. Glycobiology. 2010;20:1117–26.
Deguine V, Menasche M, Ferrari P, Fraisse L, Pouliquen Y, Robert L. Free radical depolymerization of hyaluronan by Maillard reaction products: role in liquefaction of aging vitreous. Int J Biol Macromol. 1998;22:17–22.
Wheeler-Jones CP, Farrar CE, Pitsillides AA. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Curr Opin Investig Drugs. 2010;11:997–1006.
Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25 + CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66.
• Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. This study demonstrates some of the challenges and the potential of examining insulitis in human cadaveric specimens. Similar studies examining the ECM and other components of the islet inflammatory milieu are underway.
Badami E, Sorini C, Coccia M, Usuelli V, Molteni L, Bolla AM, et al. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes. 2011;60:2120–4.
Herrath J, Muller M, Amoudruz P, Janson P, Michaelsson J, Larsson PT, et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur J Immunol. 2011;41:2279–90.
• Wesley JD, Sather BD, Perdue NR, Ziegler SF, Campbell DJ. Cellular requirements for diabetes induction in DO11.10xRIPmOVA mice. J Immunol. 2010;185:4760–8. This study demonstrates the presence of Foxp3+ regulatory T-cells in autoimmune insulitis and shows that these Treg function well ex vivo, implicating a role for the tissue environment in their evident dysfunction in vivo.
Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97.
Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–15.
Vukmanovic-Stejic M, Zhang Y, Akbar AN, Macallan DC. Measurement of proliferation and disappearance of regulatory T cells in human studies using deuterium-labeled glucose. Methods Mol Biol. 2011;707:243–61.
• Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J Immunol. 2010;184:4733–40. This study and others reporting the same finding with human Treg offers a possible mechanism for how sterile injury could lead to Treg dysfunction.
Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–27.
Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, et al. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4 + CD25+ regulatory T cells. J Immunol. 2007;179:744–7.
Liu T, Soong L, Liu G, Konig R, Chopra AK. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol Direct. 2009;4:40.
Asari A, Kanemitsu T, Kurihara H. Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J Biol Chem. 2010;285:24751–8.
Huang TL, Hsu HC, Yang KC, Lin FH. Hyaluronan up-regulates IL-10 expression in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Orthop Res. 2011;29:495–500.
Larkin J, Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. CD44 differentially activates mouse NK T cells and conventional T cells. J Immunol. 2006;177:268–79.
Vernon RB, Preisinger A, Gooden MD, D'Amico LA, Yue BB, Bollyky PL, et al. Reversal of diabetes in mice with a bioengineered islet implant incorporating a Type I collagen hydrogel and sustained release of vascular endothelial growth factor. Cell Transplant. 2012. doi:10.3727/096368911X636786.
Parnaud G, Hammar E, Ribaux P, Donath MY, Berney T, Halban PA. Signaling pathways implicated in the stimulation of β-cell proliferation by extracellular matrix. Mol Endocrinol. 2009;23:1264–71.
• Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12. This review is a good introduction to what is known about the ECM in healthy islets.
Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng B Rev. 2011;17:235–47.
Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human β-cells: effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55:2723–9.
Wang RN, Rosenberg L. Maintenance of β-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181–90.
Van Deijnen JH, van Suylichem PT, Wolters GH, van Schilfgaarde R. Distribution of collagens type I, type III and type V in the pancreas of rat, dog, pig and man. Cell Tissue Res. 1994;277:115–21.
Kragl M, Lammert E. Basement membrane in pancreatic islet function. Adv Exp Med Biol. 2010;654:217–34.
Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, et al. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51:1680–8.
Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 2010;31:1676–82.
Jalili RB, Moeen RA, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol. 2011;226:1813–9.
Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, et al. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 2009;383:113–8.
• Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132–41. This is a well done study that raising intriguing possibilities regarding the rolw of ECM in islet homeostasis.
Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for β-cell growth and differentiation. Diabetes Obes Metab. 2008;10 Suppl 4:119–27.
Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, et al. Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci U S A. 2000;97:285–90.
Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–7.
Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–72.
Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73.
Debussche X, Lormeau B, Boitard C, Toublanc M, Assan R. Course of pancreatic β-cell destruction in prediabetic NOD mice: a histomorphometric evaluation. Diabete Metab. 1994;20:282–90.
Calafiore R, Pietropaolo M, Basta G, Falorni A, Picchio ML, Brunetti P. Pancreatic β-cell destruction in non-obese diabetic mice. Metabolism. 1993;42:854–9.
Reddy S, Bradley J. Immunohistochemical demonstration of nitrotyrosine, a biomarker of oxidative stress, in islet cells of the NOD mouse. Ann N Y Acad Sci. 2004;1037:199–202.
Estella E, McKenzie MD, Catterall T, Sutton VR, Bird PI, Trapani JA, et al. Granzyme B-mediated death of pancreatic β-cells requires the proapoptotic BH3-only molecule bid. Diabetes. 2006;55:2212–9.
Papaccio G, Linn T, Federlin K, Volkman A, Esposito V, Mezzogiorno V. Further morphological and biochemical observations on early low dose streptozocin diabetes in mice. Pancreas. 1991;6:659–67.
Rakus JF, Mahal LK. New technologies for glycomic analysis: toward a systematic understanding of the glycome. Annu Rev Anal Chem (Palo Alto Calif). 2011;4:367–92.
•• Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11:M111. These authors outline a novel and comprehensive approach to characterization of the ECM in different tissues that will be useful in T1D studies.
Acknowledgments
This work was supported by National Institutes of Health grants DK046635 (to G.T.N.); DK080178, DK089128 and U01AI101984 (to P.L.B.); and HL018645 and a BIRT supplement AR037296 (to T.N.W.). This work was also supported by grants from the Juvenile Diabetes Research Foundation (nPOD 25-2010-648 (to T.N.W.), and The Center for Translational Research at BRI (to G.T.N.).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bollyky, P.L., Bogdani, M., Bollyky, J.B. et al. The Role of Hyaluronan and the Extracellular Matrix in Islet Inflammation and Immune Regulation. Curr Diab Rep 12, 471–480 (2012). https://doi.org/10.1007/s11892-012-0297-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0297-0