Abstract
Unfortunately, the only approved medical treatment for type 1 diabetes mellitus (DM) is insulin, despite the fact that tight control cannot be reached without some serious side effects such as hypoglycemia and weight gain. More and more importance is now shifted towards developing new drugs that can reach a better glycemic control with lesser side effects. Some of these promising drugs are the glucagon-like peptides 1 (GLP-1) and their agonists, which have been FDA approved for the treatment of type 2 DM. The purpose of this article is to review all of the relevant literature on the potential role of GLP-1 in the treatment of type 1 DM. The major source of data acquisition included Medline search strategies, using the words “type 1 diabetes mellitus” and “GLP-1.” Articles published in the last 20 years were screened. GLP-1 increases insulin secretion in humans with existing beta cells; it also decreases glucagon secretion, and blunts appetite. Of note, new animal studies demonstrate a role in beta cell-proliferation and decreased apoptosis. Because of all the effects mentioned above, GLP-1 seems to be a promising drug for type 1 DM treatment, but more studies are still needed before solid conclusions can be drawn.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Bayliss WM, Starling EH. Mechanism of pancreatic secretion. J Physiol. 1902;28:235–334.
Loew ER, Gray JS, Ivy AC. Is a duodenal hormone involved in carbohydrate metabolism? Am J Physiol. 1940;129:659–63.
Loew ER, Gray JS, Ivy AC. The effect of duodenal instillation of hydrochloric acid upon the fasting blood sugar of dogs. Am J Physiol. 1939;126:270–6.
Loew ER, Gray JS, Ivy AC. The effect of acid stimulation of the duodenum upon experimental hyperglycemia and utilization of glucose. Am J Physiol. 1940;128:298–308.
Elrick H, Stimmler L, Hlad CJ, Arai Y. Plasma insulin responses to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82.
Mcintryre N, Holdsworth CD, Turner DA. New interpretation of oral glucose tolerance. Lancet. 1964;II:20–1.
Brown JC. A gastric inhibitory polypeptide. I. The amino acid composition and the tryptic peptides. Can J Biochem. 1971;49:255–61.
Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971;49:867–72.
Brown JC, Mutt V, Pederson RA. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol. 1970;209:57–64.
Dupreo J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–28.
Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–71.
Schmidt WE, Siegel EG, Creutzfeldt W. Glucagonlike peptide-1 but not glucagon-like-peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–7.
Mojsov S, Weir GC, Habener JF. Insulinotropin glucagon-like peptide-1 (7–36): co-encoded in the glucagons gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–19.
Meier JJ, Hucking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes. 2001;50:2497–504.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–16.
Drucker DJ, Yusta B, Boushey RP, Deforest L, Brubaker PL. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–91.
Rocca AS, Lagreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142:1148–55.
Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605–11.
Sakurai H, Dobbs RE, Unger RH. The effect of somatostatin on the response of GLI to the intraduodenal administration of glucose, protein, and fat. Diabetologia. 1975;11:427–30.
Matsuyama T, Hoffman WH, Dunbar JC, Foa NL, Foa PP. Glucose, insulin, pancreatic glucagon and glucagon-like immunoreactive materials in the plasma of normal and diabetic children. Effect of the initial insulin treatment. Horm Metab Res. 1975;7:452–6.
Balks HJ, Holst JJ. Von Zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82:786–90.
Dumoulin V, Dakka T, Plaisancie P, Chayvialle JA, Cuber JC. Regulation of glucagon-like peptide-1-(7-36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology. 1995;136:5182–88.
Lickley HL, Kemmer FW, Gray DE, et al. Chromatographic pattern of extrapancreatic glucagons and glucagon-like immunoreactivity before and during stimulation by epinephrine and participation of glucagons in epinephrine-induced hepatic glucose overproduction. Surgery. 1981;90:186–94.
Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. J Biol Chem. 1992;267:7402–5.
Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750–4.
Madsbad S, Krarup T, Regeur L, Faber OK, Binder C. Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinol (Copenh). 1980;95:359–63.
Madsbad S. Prevalence of residual B cell function and its metabolic consequences in Type 1 (insulin-dependent) diabetes. Diabetologia. 1983;24:141–7.
Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–43.
Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51:951–7.
Lugari R, Dell’Anna C, Ugolotti D, et al. Effect of nutrient ingestion on glucagon-like peptide 1 (7-36 amide) secretion in human type 1 and type 2 diabetes. Horm Metab Res. 2000;32:424–8.
Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13.
• Varga T, Firneisz G, Nagy G, Somogyi A. Elevated serum DPP4 activity in type 1 diabetes mellitus: a direct comparison. Orv Hetil 2010 151: 899-902. This study showed that compared with type 2 DM and normal controls, patients with type 1 DM had an elevated DPP4 activity that can affect GLP-1 activity.
Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I (7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–6.
Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117.
Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–22.
Dupre J, Behme MT, Hramiak IM, et al. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes. 1995;44:626–30.
Dupre J, Behme MT, Hramiak IM, McDonald TJ. Subcutaneous glucagon-like peptide I combined with insulin normalizes postcibal glycemic excursions in IDDM. Diabetes Care. 1997;20:381–4.
Behme MT, Dupre J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;10:1–9.
•• Raman VS, Mason KJ, Rodriguez LM, Hassan K, Yu Xi, Bomgaars L, et al. The role of adjunctive exenatide therapy in pediatrics type 1 DM. Diabetes Care 2010;33(6):1294–6. This randomized controlled trial showed that compared with insulin monotherapy treatment with 2 different doses of exenatide (both low and high dose) led to a better controlled postprandial hyperglycemia in adolescent patients with type 1 DM.
• Kielgast A, Madsbad H. Effect of GLP-1 on alfa and beta-cell function in c-peptide negative type 1 diabetic patients. J Clin Endocrin Metab 2010;95(5):2492–6. In this study done on C-peptide negative type 1 DM patients, GLP-1 agonists decreased both non-stimulated and arginine-stimulated glucagon release.
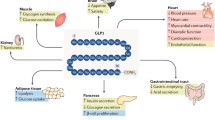
Villanueva-Penacarrillo ML, Alcantara AI, Clemente F, Delgado E, Valverde I. Potent glycogenic effect of GLP-1(7-36)amide in rat skeletal muscle. Diabetologia. 1994;37:1163–6.
Merida E, Delgado E, Molina LM, Villanueva-Penacarrillo ML, Valverde I. Presence of glucagon and glucagon-like peptide-1-(7-36) amide receptors in solubilized membranes of human adipose tissue. J Clin Endocrinol Metab. 1993;77:1654–7.
Villanueva-Penacarrillo ML, Delgado E, Trapote MA, Alcantara A, Clemente F, Luque MA, et al. Glucagon-like peptide-1 binding to rat hepatic membranes. J Endocrinol. 1995;46:183–9.
Morales M, Lopez-Delgado MI, Alcantara AI, Luque MA, Clemente F, Marquez L, et al. Preserved GLP-1 effects on glycogen synthase a activity and glucose metabolism in isolated hepatocytes and skeletal muscle from diabetic rats. Diabetes. 1997;46:1264–9.
Lopez-Delgado MI, Morales M, Villanueva-Penacarrillo ML, Malaisse WJ, Valverde I. Effects of glucagon-like peptide 1 on the kinetics of glycogen synthase a in hepatocytes from normal and diabetic rats. Endocrinology. 1998;139:2811–17.
Meneilly GS, McIntosh CH, Pederson RA, et al. Effect of glucagon- like peptide 1 (7-36 amide) on insulin-mediated glucose uptake in patients with type 1 diabetes. Diabetes Care. 2003;26:837–42.
Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk FW, et al. Effect of glucagon-like peptide-1(7-36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes. 2001;50:565–72.
Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA. Lack of effect of exendin-4 and glucagon-like peptide-1-(7-36)-amide on insulin action in nondiabetic humans. Diabetologia. 2002;45:1410–15.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57.
Brubaker PL, Drucker DJ. Minireview. Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–9.
Deacon CF. Therapeutic strategies based on glucagon-like peptide. Diabetes. 2004;53:2181–9.
Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–23.
List JF, Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2004;286:E875–81.
Scrocchi LA, Brown TJ, MacLusky N, Brubaker PL, Auerbach AB, Joyner AL, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2:1254–8.
Serre V, Dolci W, Schaerer E, Scrocchi L, Drucker D, Efrat S, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon- like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3,5-monophosphate levels and beta-cell glucose competence. Endocrinology. 1998;139:4448–54.
Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;01:1421–30.
Fehmann HC, Habener JF. Insulinotropic hormone glucagon- like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–66.
Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99:2883–9.
Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–83.
Anderson MS, Bluestone JA. The NOD mouse. A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85.
Zhang J, Tokui Y, Yamagata K, et al. Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia. 2007;50:1900–9.
Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008;149:1338–49.
Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57:3281–8.
Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with Anti-CD3 monoclonal antibody by enhancing recovery of β-cells. Endocrinology. 2007;148:5136–44.
Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun. 2006;344:1017–22.
Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–5.
•• Perez-Arana G, Blandino-Rosano M, Prada-Oliveira A, Aguilar-Diosdado M, Segundo C. Decrease in beta-cell proliferation precedes apoptosis during diabetes development in Bio-Breeding/Worcester rat: beneficial role of Exendin-4. Endocrinology. 2010;151:2538–46. In this study comparing combination of exendin-4 and monoclonal anti-INF gamma monoclonal antibody to each drug alone, it was shown that exendin-4 monotherapy decreased beta-cell apoptosis and the combination increased beta-cell proliferation better than each drug alone.
•• Liu MJ, Han J, Lee YS, Park MS, Shin S, Jun HS. Amelioration of hyperglycemia by intestinal overexpression of GLP-1 in mice. J Mol Med. 2010;88:351–8. This study showed that local intra-intestinal production of GLP-1 in diabetic mice can induce intestinal stem cell differentiation into insulin secreting cells, and showed a high insulin level with better glycemic control in these mice.
Burcelin R. EuCSGLP-1. What is known, new and controversial about GLP-1? Minutes of the 1st European GLP-1 club meeting, Marseille 28-29 May 2008. Diabetes Metab. 2008;34(6 Pt 1):627–30.
Meier JJ. Beta-cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51(5):703–13.
Roep BO. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann NY Acad Sci. 2007;1103:1–10.
Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. Beta-cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–91.
Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–8.
Suen PM, Li K, Chan JC, Leung PS. In vivo treatment with glucagon- like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol. 2006;38:951–60.
Froud T, Faradji RN, Pileggi A, et al. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy and metabolic effects. Transplantation. 2008;86:36–45.
Ghofaili KA, Fung M, Ao Z, et al. Effect of exenatide on beta-cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;15(83):24–8.
Faradji RN, Tharavanij T, Messinger S, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;27(86):1658–65.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Issa, C.M., Azar, S.T. Possible Role of GLP-1 and Its Agonists in the Treatment of Type 1 Diabetes Mellitus. Curr Diab Rep 12, 560–567 (2012). https://doi.org/10.1007/s11892-012-0291-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0291-6