Abstract
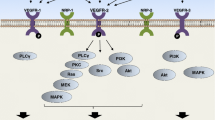
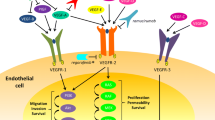
Vascular endothelial growth factor (VEGF)—over-expressed in colorectal cancer—is associated with disease progression and inferior survival. Based on successful, randomized, phase III trials, anti-VEGF therapeutics have entered clinical practice. Bevacizumab, a VEGF-specific antibody, was the first anti-angiogenic agent to be approved by the Food and Drug Administration to be used in combination with standard chemotherapy in the first and second line of treatment in metastatic colorectal cancer. VEGF-targeted therapy may lead to indirect killing of cancer cells by damaging tumor blood vessels and may increase the radiosensitivity of tumor-associated endothelial cells. VEGF blockade can also normalize tumor vasculature thereby leading to greater tumor oxygenation (a known radiosensitizer) and drug penetration. A dose-escalation phase I trial has demonstrated that the combination of bevacizumab at the 5-mg/kg dose with radiochemotherapy was well tolerated by patients with rectal cancers. In addition, results from an array of correlative studies have demonstrated that bevacizumab has antivascular effects and supported the normalization hypothesis. The ongoing phase II study will further elucidate the mechanisms of action and efficacy of bevacizumab in locally advanced rectal cancer.
Similar content being viewed by others
References and Recommended Reading
Chin KF, Greenman J, Gardiner E, et al.: Preoperative serum vascular endothelial growth factor can select patients for adjuvant treatment after curative resection in colorectal cancer. Br J Cancer 2000, 83:1425–1431.
Hyodo I, Doi T, Endo H, et al.: Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer 1998, 34:2041–2045.
Nanashima A, Ito M, Sekine I, et al.: Significance of angiogenic factors in liver metastatic tumors originating from colorectal cancers. Dig Dis Sci 1998, 43:2634–2640.
Cascinus S, Graziano F, Catalano V, et al.: Vascular endothelial growth factor (VEGF), p53, and BAX expression in node positive rectal cancer. Pro Am Soc Clin Oncol 2001, 20:595.
Kabbinavar F, Hurwitz H, Fehrenbacher L, et al.: Phase II trial comparing rhuMAB VEGF (recombinant humanized monoclonal antibody to vascular endothelial growth factor) plus 5-fluoroouracil/leucovorin (FU/LV) to FU/LV) alone in patients with metastatic colorectal cancer. J Clin Oncol 2003, 21:60–65.
Hurwitz H, Fehrenbacher L, Novotny W, et al.: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med 2004, 350:2335–2342.
Giantonio BJ, Levy DE, O’Dwyer PJ, et al.: A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 2006, 17:1399–1403.
National Cancer Institute: Bevacizumab (Avastin ™) for Metastatic Colorectal Cancer. http://www.cancer.gov/newscenter/pressreleases/bevacizumab. Accessed February 2, 2007.
Lee CG, Heijn M, di Tomaso E, et al.: Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 2000, 60:5565–5570.
Kozin SV, Boucher Y, Hicklin DJ, et al.: Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenograts. Cancer Res 2001, 61:39–44.
Winkler F, Kozin S, Tong R, et al.: Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metallproteinases. Cancer Cell 2004, 6:553–562.
Tong RT, Boucher Y, Kozin SV, et al.: Vascular normalization by VEGFR2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004, 64:3731–3736.
Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature Med 2001, 7:987–989.
Jain RK: Normalization of tumor vasculature: an emerging concept in anti-angiogenic therapy. Science 2005, 307:58–62.
Willett CG, Boucher Y, di Tomaso E, et al.: Direct evidence that the VEGF-specific antibody bevacizumab has anti-vascular effects in human rectal cancer. Nature Med 2004, 10:145–147.
Willett CG, Boucher Y, Duda DG, et al.: Surrogate markers for antiangiogenic therapy and dose limiting toxicities for Bevacizumab with radio-chemotherapy: continued experience of a Phase I trial in rectal cancer patients. J Clin Oncol 2005, 23:8136–8139.
Duda, DG, Cohen KS, di Tomaso E, et al.: Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for anti-angiogenic therapy. J Clin Oncol 2006, 24:1449–1453.
Drevs J, Medinger M, Mross K, et al.: Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors [abstract]. J Clin Oncol 2005, 23(Suppl):3002.
Willett CG, Duda DG, di Tomaso E, et al.: Complete pathological response in T4 locally advanced rectal cancer after treatment with bevacizumab and standard radiochemotherapy. Nat Clin Pract Oncol 2007, In press.
Motzer RJ, Michaelson MD, Redman BG, et al.: Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006, 24:16–24.
Dowlati A, Gray R, Johnson DH, et al.: Prospective correlative assessment of biomarkers in E4599 randomized phase II/III trial of carboplatin and paclitaxel ± bevacizumab in advanced non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol 2006, 24(Suppl):7027.
Batchelor TT, Sorensen AG, di Tomaso E, et al.: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11:83–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willett, C.G., Duda, D.G. & Jain, R.K. Surrogate biomarkers for anti-angiogenic therapy for advanced colorectal cancer. Curr colorectal cancer rep 3, 94–98 (2007). https://doi.org/10.1007/s11888-007-0007-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-007-0007-5