Abstract
Purpose of Review
Current non-invasive tests for evaluating patients with peripheral artery disease (PAD) have significant limitations for early detection and management of patients with PAD and are generally focused on the evaluation of large vessel disease. PAD often involves disease of microcirculation and altered metabolism. Therefore, there is a critical need for reliable quantitative non-invasive tools that can assess limb microvascular perfusion and function in the setting of PAD.
Recent Findings
Recent developments in positron emission tomography (PET) imaging have enabled the quantification of blood flow to the lower extremities, the assessment of the viability of skeletal muscles, and the evaluation of vascular inflammation and microcalcification and angiogenesis in the lower extremities. These unique capabilities differentiate PET imaging from current routine screening and imaging methods.
Summary
The purpose of this review is to highlight the promising role of PET in the early detection and management of PAD providing a summary of the current preclinical and clinical research related to PET imaging in patients with PAD and related advancement of PET scanner technology.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet Lond Engl. 2013;382(9901):1329–40. https://doi.org/10.1016/S0140-6736(13)61249-0.
Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. https://doi.org/10.1161/CIR.0000000000000350.
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26. https://doi.org/10.1161/CIRCRESAHA.116.303849.
Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24. https://doi.org/10.1001/jama.286.11.1317.
Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151(11):1070–7. https://doi.org/10.1001/jamasurg.2016.2018.
Teraa M, Conte MS, Moll FL, et al. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5(2):e002938. https://doi.org/10.1161/JAHA.115.002938.
• Misra S, Shishehbor MH, Takahashi EA, et al. Perfusion assessment in critical limb ischemia: principles for understanding and the development of evidence and evaluation of devices: a scientific statement from the American Heart Association. Circulation. 2019;140(12):e657–72. https://doi.org/10.1161/CIR.0000000000000708. This scientific statement discusses various noninvasive assessments of limb perfusion in critical limb ischemia (CLI), addressing limitations and opportunities for improvement.
Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–7. https://doi.org/10.1111/j.1742-481X.2007.00392.x.
Beckman JA, Duncan MS, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140(6):449–58. https://doi.org/10.1161/CIRCULATIONAHA.119.040672.
Bowling FL, Rashid ST, Boulton AJM. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015;11(10):606–16. https://doi.org/10.1038/nrendo.2015.130.
Howard DPJ, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132(19):1805–15. https://doi.org/10.1161/CIRCULATIONAHA.115.016424.
Stein R, Hriljac I, Halperin JL, et al. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med Lond Engl. 2006;11(1):29–33. https://doi.org/10.1191/1358863x06vm663oa.
Englund EK, Langham MC, Li C, et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson. 2013;15(1):70. https://doi.org/10.1186/1532-429X-15-70.
Utz W, Jordan J, Niendorf T, et al. Blood oxygen level–dependent MRI of tissue oxygenation. Arterioscler Thromb Vasc Biol. 2005;25(7):1408–13. https://doi.org/10.1161/01.ATV.0000170131.13683.d7.
Stacy MR, Qiu M, Papademetris X, et al. Application of BOLD magnetic resonance imaging for evaluating regional volumetric foot tissue oxygenation: a feasibility study in healthy volunteers. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2016;51(5):743–9. https://doi.org/10.1016/j.ejvs.2016.02.008.
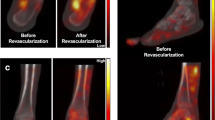
Chou T-H, Alvelo JL, Janse S, et al. Prognostic value of radiotracer-based perfusion imaging in critical limb ischemia patients undergoing lower extremity revascularization. JACC Cardiovasc Imaging. 2021;14(8):1614–24. https://doi.org/10.1016/j.jcmg.2020.09.033.
Chou T-H, Janse S, Sinusas AJ, et al. SPECT/CT imaging of lower extremity perfusion reserve: a non-invasive correlate to exercise tolerance and cardiovascular fitness in patients undergoing clinically indicated myocardial perfusion imaging. J Nucl Cardiol. 2020;27(6):1923–33. https://doi.org/10.1007/s12350-019-02019-w.
Burchert W, Schellong S, van den Hoff J, et al. Oxygen-15-water PET assessment of muscular blood flow in peripheral vascular disease. J Nucl Med Off Publ Soc Nucl Med. 1997;38(1):93–8.
Schmidt MA, Chakrabarti A, Shamim-Uzzaman Q (Afifa), et al. Calf flow reserve with H215O PET as a quantifiable index of lower extremity flow. J Nucl Med 2003;44(6):915–9.
Scremin OU, Figoni SF, Norman K, et al. Preamputation evaluation of lower-limb skeletal muscle perfusion with H(2) (15)O positron emission tomography. Am J Phys Med Rehabil. 2010;89(6):473–86. https://doi.org/10.1097/PHM.0b013e3181d89b08.
Peñuelas I, Aranguren XL, Abizanda G, et al. (13)N-ammonia PET as a measurement of hindlimb perfusion in a mouse model of peripheral artery occlusive disease. J Nucl Med Off Publ Soc Nucl Med. 2007;48(7):1216–23. https://doi.org/10.2967/jnumed.106.039180.
Scholtens AM, Tio RA, Willemsen A, et al. Myocardial perfusion reserve compared with peripheral perfusion reserve: a [13N]ammonia PET study. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2011;18(2):238–46. https://doi.org/10.1007/s12350-011-9339-2.
Liu Z, Thorn S, Wu J, et al. Assessment of lower extremities flow using dynamic Rb-82 PET: acquisition protocols and quantification methods. J Nucl Med. 2021;62(supplement 1):53–53.
Moody JB, Hiller KM, Lee BC, et al. The utility of 82Rb PET for myocardial viability assessment: Comparison with perfusion-metabolism 82Rb-18F-FDG PET. J Nucl Cardiol. 2019;26(2):374–86. https://doi.org/10.1007/s12350-019-01615-0.
Arumugam P, Tout D, Tonge C. Myocardial perfusion scintigraphy using rubidium-82 positron emission tomography. Br Med Bull. 2013;107(1):87–100. https://doi.org/10.1093/bmb/ldt026.
Patel KK, Singh A, Bateman TM. The potential of F-18 flurpiridaz PET/CT myocardial perfusion imaging for precision imaging. Curr Cardiol Rep. 2022;24(8):987–94. https://doi.org/10.1007/s11886-022-01713-5.
Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115(7):1699–702. https://doi.org/10.1172/JCI25758.
Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med Lond Engl. 2000;5(1):55–9. https://doi.org/10.1177/1358836X0000500109.
Koh H-CE, van Vliet S, Meyer GA, et al. Heterogeneity in insulin-stimulated glucose uptake among different muscle groups in healthy lean people and people with obesity. Diabetologia. 2021;64(5):1158–68. https://doi.org/10.1007/s00125-021-05383-w.
Pande RL, Park M-A, Perlstein TS, et al. Impaired skeletal muscle glucose uptake by [18F]fluorodeoxyglucose–positron emission tomography in patients with peripheral artery disease and intermittent claudication. Arterioscler Thromb Vasc Biol. 2011;31(1):190–6. https://doi.org/10.1161/ATVBAHA.110.217687.
Tashiro M, Fujimoto T, Itoh M, et al. 18F-FDG PET imaging of muscle activity in runners. J Nucl Med Off Publ Soc Nucl Med. 1999;40(1):70–6.
Pappas GP, Olcott EW, Drace JE. Imaging of skeletal muscle function using 18FDG PET: force production, activation, and metabolism. J Appl Physiol. 2001;90(1):329–37. https://doi.org/10.1152/jappl.2001.90.1.329.
Rudroff T, Kindred JH, Benson J-M, et al. Greater glucose uptake heterogeneity in knee muscles of old compared to young men during isometric contractions detected by [18F]-FDG PET/CT. Front Physiol. 2014;5. https://doi.org/10.3389/fphys.2014.00198.
Trombella S, García D, Colin DJ, et al. [11C]acetate and PET/CT assessment of muscle activation in rat studies. Int J Comput Assist Radiol Surg. 2016;11(5):733–43. https://doi.org/10.1007/s11548-015-1260-8.
van Hall G, Sacchetti M, Rådegran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. J Physiol. 2002;542(Pt 1):263–72. https://doi.org/10.1113/jphysiol.2001.014340.
Buchegger F, Ratib O, Willi J-P, et al. [11C]acetate PET/CT visualizes skeletal muscle exercise participation, impaired function, and recovery after hip arthroplasty; first results. Mol Imaging Biol. 2011;13(4):793–9. https://doi.org/10.1007/s11307-010-0415-9.
de Boer SA, Hovinga-de Boer MC, Heerspink HJL, et al. Arterial stiffness is positively associated with 18F-fluorodeoxyglucose positron emission tomography-assessed subclinical vascular inflammation in people with early type 2 diabetes. Diabetes Care. 2016;39(8):1440–7. https://doi.org/10.2337/dc16-0327.
Fernández-Friera L, Fuster V, López-Melgar B, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol. 2019;73(12):1371–82. https://doi.org/10.1016/j.jacc.2018.12.075.
Ishii H, Nishio M, Takahashi H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32(14):2337–47. https://doi.org/10.1016/j.clinthera.2010.12.001.
Keidar Z, Engel A, Hoffman A, et al. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med. 2007;48(8):1230–6. https://doi.org/10.2967/jnumed.107.040253.
Iking J, Staniszewska M, Kessler L, et al. Imaging inflammation with positron emission tomography. Biomedicines. 2021;9(2):212. https://doi.org/10.3390/biomedicines9020212.
Gourni E, Demmer O, Schottelius M, et al. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. J Nucl Med Off Publ Soc Nucl Med. 2011;52(11):1803–10. https://doi.org/10.2967/jnumed.111.098798.
Thackeray JT, Derlin T, Haghikia A, et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging. 2015;8(12):1417–26. https://doi.org/10.1016/j.jcmg.2015.09.008.
Lamare F, Hinz R, Gaemperli O, et al. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2011;52(1):33–9. https://doi.org/10.2967/jnumed.110.079038.
Pugliese F, Gaemperli O, Kinderlerer AR, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol. 2010;56(8):653–61. https://doi.org/10.1016/j.jacc.2010.02.063.
• Reijrink M, de Boer SA, Te Velde-Keyzer CA, et al. [18F]FDG and [18F]NaF as PET markers of systemic atherosclerosis progression: a longitudinal descriptive imaging study in patients with type 2 diabetes mellitus. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2022;29(4):1702–9. https://doi.org/10.1007/s12350-021-02781-w. There is a strong correlation baseline 18F-FDG uptake and 5-year follow-up 18F-NaF uptake suggesting that FDG could play a crucial role in the early detection of PAD.
Takx RAP, van Asperen R, Bartstra JW, et al. Determinants of 18F-NaF uptake in femoral arteries in patients with type 2 diabetes mellitus. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2021;28(6):2700–5. https://doi.org/10.1007/s12350-020-02099-z.
Chou T-H, Rimmerman ET, Patel S, et al. Vessel-by-vessel analysis of lower extremity 18F-NaF PET/CT imaging quantifies diabetes- and chronic kidney disease-induced active microcalcification in patients with peripheral arterial disease. EJNMMI Res. 2023;13(1):3. https://doi.org/10.1186/s13550-023-00951-0.
• Chowdhury MM, Tarkin JM, Albaghdadi MS, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease. JACC Cardiovasc Imaging. 2020;13(4):1008–17. https://doi.org/10.1016/j.jcmg.2019.03.031. The uptake of both 18F-FDG and 18F-NaF at baseline and post-intervention may assist in predicting restenosis in PAD patients undergoing percutaneous transluminal angioplasty.
Oliveira BL, Blasi F, Rietz TA, et al. Multimodal molecular imaging reveals high target uptake and specificity of 111In- and 68Ga-labeled fibrin-binding probes for thrombus detection in rats. J Nucl Med Off Publ Soc Nucl Med. 2015;56(10):1587–92. https://doi.org/10.2967/jnumed.115.160754.
Oliveira BL, Caravan P. Peptide-based fibrin-targeting probes for thrombus imaging. Dalton Trans Camb Engl. 2017;46(42):14488–508. https://doi.org/10.1039/c7dt02634j.
Lohrke J, Siebeneicher H, Berger M, et al. 18F-GP1, a novel PET tracer designed for high-sensitivity, low-background detection of thrombi. J Nucl Med Off Publ Soc Nucl Med. 2017;58(7):1094–9. https://doi.org/10.2967/jnumed.116.188896.
Goggi JL, Haslop A, Boominathan R, et al. Imaging the proangiogenic effects of cardiovascular drugs in a diabetic model of limb ischemia. Contrast Media Mol Imaging. 2019;2019:2538909. https://doi.org/10.1155/2019/2538909.
Orbay H, Hong H, Koch JM, et al. Pravastatin stimulates angiogenesis in a murine hindlimb ischemia model: a positron emission tomography imaging study with 64Cu-NOTA-TRC105. Am J Transl Res. 2013;6(1):54–63.
Wei L, Ye Y, Wadas TJ, et al. (64)Cu-labeled CB-TE2A and diamsar-conjugated RGD peptide analogs for targeting angiogenesis: comparison of their biological activity. Nucl Med Biol. 2009;36(3):277–85. https://doi.org/10.1016/j.nucmedbio.2008.12.008.
Liu Y, Pressly ED, Abendschein DR, et al. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med Off Publ Soc Nucl Med. 2011;52(12):1956–63. https://doi.org/10.2967/jnumed.111.089581.
Hedhli J, Slania SLL, Płoska A, et al. Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice. Sci Rep. 2018;8(1):5401. https://doi.org/10.1038/s41598-018-23372-9.
Cheng R, Ma J. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord. 2015;16(1):67–75. https://doi.org/10.1007/s11154-015-9310-7.
Fallavollita JA, Heavey BM, Luisi AJ, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63(2):141–9. https://doi.org/10.1016/j.jacc.2013.07.096.
Tack CJ, van Gurp PJ, Holmes C, et al. Local sympathetic denervation in painful diabetic neuropathy. Diabetes. 2002;51(12):3545–53. https://doi.org/10.2337/diabetes.51.12.3545.
Franzius C, Hermann K, Weckesser M, et al. Whole-body PET/CT with 11C-meta-hydroxyephedrine in tumors of the sympathetic nervous system: feasibility study and comparison with 123I-MIBG SPECT/CT. J Nucl Med. 2006;47(10):1635–42.
Sinusas AJ, Lazewatsky J, Brunetti J, et al. Biodistribution and radiation dosimetry of LMI1195: first-in-human study of a novel 18F-labeled tracer for imaging myocardial innervation. J Nucl Med Off Publ Soc Nucl Med. 2014;55(9):1445–51. https://doi.org/10.2967/jnumed.114.140137.
Zhang J, Maniawski P, Knopp MV. Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. 2018;8(1):97. https://doi.org/10.1186/s13550-018-0448-7.
• Badawi RD, Shi H, Hu P, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60(3):299–303. https://doi.org/10.2967/jnumed.119.226498. The EXPLORER, the first PET scanner that can scan the entire body, has the ability to conduc tcomprehensive pharmacokinetic studies, which could potentially aid in the assessment of peripheral artery disease.
Beyer T, Townsend DW, Czernin J, et al. The future of hybrid imaging—part 2: PET/CT. Insights Imaging. 2011;2(3):225–34. https://doi.org/10.1007/s13244-011-0069-4.
Rahmim A, Lodge MA, Karakatsanis NA, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46(2):501–18. https://doi.org/10.1007/s00259-018-4153-6.
Funding
Alaa Alashi and Billy C. Vermillion report an NIH training grant (NIH-NRSA T32:HL 098069). Albert J. Sinusas reports NIH funding related to this article (R01 HL163640).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Albert J. Sinusas reports Institutional grants from Jubilant and Siemens; individual consulting fees unrelated to PET imaging compounds from MicroVide, LLC; patents related to SPECT imaging agent RP805 for MicroVide, LLC; member Cardiovascular Council of SNMMI (no funds received); receipt of equipment, materials, drugs, or other services from Lantheus (MTA LMI1195) and Jubilant (Rb-82 generator). The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies and experiments involving human or animal subjects conducted by the authors mentioned in this article have been previously published and adhered to all relevant ethical standards, including the Helsinki Declaration and its amendments, institutional and national research committee norms, as well as international, national, and institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alashi, A., Vermillion, B.C. & Sinusas, A.J. The Potential Role of PET in the Management of Peripheral Artery Disease. Curr Cardiol Rep 25, 831–839 (2023). https://doi.org/10.1007/s11886-023-01904-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01904-8