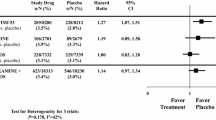
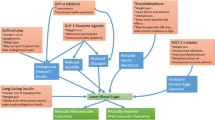
Abstract
Purpose of Review
This review summarizes recent cardiovascular outcome trials (CVOTs) with glucose-lowering drugs.
Recent Findings
The majority of recent CVOTs with glucose-lowering drugs have tested dipeptidyl peptidase-4 inhibitors (DPP4-i), glucagon-like peptide-1 receptors agonists (GLP1-RA), and sodium-glucose cotransporter 2 inhibitors (SGLT2i), but studies have also been performed with other agents including thiazolidinediones and insulin.
Summary
All CVOTs with DPP4-I, GLP1-RA, and SGLT2-i have demonstrated the cardiovascular (CV) safety of these agents compared to usual care. However, certain GLP1-RAs (liraglutide, subcutaneous semaglutide, albiglutide, dulaglutide) and SGLT2-i (empagliflozin, canagliflozin) have demonstrated a CV benefit, showing significant reductions in composite cardiovascular outcomes. Furthermore, all SGLT2-i also significantly decreased the risk for hospitalization for heart failure. Results from these studies have altered clinical guidelines worldwide and have resulted in new indications for some glucose-lowering drugs. In patients with T2D and high risk for CVD, GLP-1RA or SGLT2-i with proven cardiovascular benefit are recommended, irrespective of glycemic control.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–13.
Atlas IDFD. Individual, social and economic impact. 2019. Available from: https://diabetesatlas.org/en/sections/individual-social-and-economic-impact.html. Accessed 26 Oct 2020.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53.
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol. 2014;2(10):793–800.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
White JR. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82–6.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J. 2008;155(1):26–32 e1-6.
Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. Design of DEVOTE (Trial Comparing Cardiovascular Safety of Insulin Degludec vs Insulin Glargine in Patients With Type 2 Diabetes at High Risk of Cardiovascular Events) - DEVOTE 1. Am Heart J. 2016;179:175–83.
Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–32.
Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Dargie H, et al. Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocol. Diabetologia. 2005;48(9):1726–35.
Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes — an interim analysis. N Engl J Med. 2007;357(1):28–38.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35.
Viscoli CM, Brass LM, Carolei A, Conwit R, Ford GA, Furie KL, et al. Pioglitazone for secondary prevention after ischemic stroke and transient ischemic attack: rationale and design of the Insulin Resistance Intervention after Stroke Trial. Am Heart J. 2014;168(6):823–9.e6.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42.
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. Jama. 2019;321(1):69–79.
Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®). Diab Vasc Dis Res. 2015;12(3):164–74.
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155–66.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57.
Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166(5):823–30.e5.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39.
Holman RR, Bethel MA, George J, Sourij H, Doran Z, Keenan J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103–10.
Green JB, Hernandez AF, D’Agostino RB, Granger CB, Janmohamed S, Jones NP, et al. Harmony Outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus-Rationale, design, and baseline characteristics. Am Heart J. 2018;203:30–8.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20(1):42–9.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51.
Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol. 2014;13:102.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)--a randomized placebo-controlled trial. Am Heart J. 2013;166(2):217–23.e11.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): A randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(3):387–93.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347–57.
Cannon CP, McGuire D, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, et al. Design and baseline characteristics of the evaluation of ERTUGLIFOZIN efficacy and safety cardiovascular outcomes trial (VERTIS-CV). J Am Coll Cardiol. 2018;71(11 Supplement):A1825.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2004967
Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1463–76.
Holman RR, Bethel MA, Chan JC, Chiasson JL, Doran Z, Ge J, et al. Rationale for and design of the Acarbose Cardiovascular Evaluation (ACE) trial. Am Heart J. 2014;168(1):23–9.e2.
Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–86.
Bilal A, Pratley RE. Cardiovascular outcomes trials update: insights from the DEVOTE Trial. Curr Diab Rep. 2018;18(11):102.
Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–18.
Yasmin S, Jayaprakash V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: from past to present. Eur J Med Chem. 2017;126:879–93.
Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A. The prospective pioglitazone clinical trial in macrovascular events (PROactive). Can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5,238 patients. Diabetes Care. 2004;27(7):1647–53.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89.
Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. 2016;18(3):266–73.
Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care. 2016;39(10):1684–92.
Young LH, Viscoli CM, Curtis JP, Inzucchi SE, Schwartz GG, Lovejoy AM, et al. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation. 2017;135(20):1882–93.
Young LH, Viscoli CM, Schwartz GG, Inzucchi SE, Curtis JP, Gorman MJ, et al. Heart failure after ischemic stroke or transient ischemic attack in insulin-resistant patients without diabetes mellitus treated with pioglitazone. Circulation. 2018;138(12):1210–20.
Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, et al. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017;102(3):914–22.
Zhou Y, Huang Y, Ji X, Wang X, Shen L, Wang Y. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: a meta-Analysis. J Clin Endocrinol Metab. 2019;105(5):1670–81.
Demuth HU, McIntosh CH, Pederson RA. Type 2 diabetes--therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta. 2005;1751(1):33–44.
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76.
McGuire DK, Van de Werf F, Armstrong PW, Standl E, Koglin J, Green JB, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(2):126–35.
Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8–16.
Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19.
Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Phys. 1997;273(5):E981–8.
. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85 This is a meta-analysis of cardiovascular outcomes with GLP-1 receptor agonists use in T2D patients.
Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12(2):78–89.
Cai X, Ji L, Chen Y, Yang W, Zhou L, Han X, et al. Comparisons of weight changes between sodium-glucose cotransporter 2 inhibitors treatment and glucagon-like peptide-1 analogs treatment in type 2 diabetes patients: A meta-analysis. J Diabetes Investig. 2017;8(4):510–7.
Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials With 22 528 Patients. J Am Heart Assoc. 2017;6. https://doi.org/10.1161/jaha.116.004007
Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, et al. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6. https://doi.org/10.1161/jaha.117.005686
FDA. STEGLATRO™ (ertugliflozin)-highlights of prescribing information; Revised: 01/2020; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209803s002lbl.pdf. Accessed 10/26/2020.
FDA. JARDIANCE® (empagliflozin)_highlights of prescribing information; Revised: 1/2020; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204629s023lbl.pdf. Accessed 10/26/2020.
FDA. FARXIGA® (dapagliflozin)_highlights of prescribing information ; Revised: 05/2020; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf. Accessed 10/26/2020.
FDA. INVOKANA (canagliflozin)_highlights of prescribing information; Revised: 08/2020; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204042s034lbl.pdf. Accessed 10/26/2020.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.4511This is a meta-analysis of cardio-renal outcomes with SGLT-2inhibitors.
FDA. Ozempic-Semaglutide-[prescribing information]; 2020. Access from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf. Revised on: 01/2020; Access Date: November 01, 2020.
FDA. Victoza- Liraglutide_Hghlights of Prescribing Information_; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022341s035lbl.pdf. Revised: 08/2020; Access Date: 11/01/2020.
FDA. Trulicity-Dulaglutide-[Prescribing Information]; 2020. Access from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s036lbl.pdf. Revised on: 09/2020; Access Date: November 1, 2020.
Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease. A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200–23.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–98.
American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–S23.
. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323 These are the 2019 ESC and EASD guidelines.
ADA. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S98–S110.
FDA. Type 2 diabetes mellitus: evaluating the safety of new drugs for improving glycemic control; Draft Guidance for Industry; Availability [Docket No. FDA–2020–N–0008] Notic on: 03/10/2020. Federal Register. 2020;85(47). https://www.federalregister.gov/documents/2020/03/10/2020-04877/type-2-diabetes-mellitus-evaluating-the-safety-of-new-drugs-for-improving-glycemic-control-draft. Date Accessed: July 07, 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Tina Thethi reports consulting fees from Novo Nordisk. She is also on the speaker bureau for Novo Nordisk.
Dr. Richard E. Pratley reports consulting fees from AstraZeneca; consulting fees from Glytec, LLC; grants from Hanmi Pharmaceutical Co.; grants and consulting fees from Janssen; grants from Lexicon Pharmaceuticals; consulting fees from Merck; consulting fees from Mundipharma; grants, speaker fees and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants from Poxel SA; grants and consulting fees from Sanofi; consulting fees from Scohia Pharma Inc.; consulting fees from Sun Pharmaceutical Industries; personal consulting fees from Sanofi US Services, Inc., outside the submitted work. Except for consulting fees in February 2018 and June 2018 from Sanofi US Services, Inc., R.E.P.’s services were paid for directly to AdventHealth, a nonprofit organization.
Dr. Anika Bilal reports no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Thethi, T.K., Bilal, A. & Pratley, R.E. Cardiovascular Outcome Trials with Glucose-Lowering Drugs. Curr Cardiol Rep 23, 75 (2021). https://doi.org/10.1007/s11886-021-01505-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01505-3