Abstract
Purpose of Review
To assess the effects of tezepelumab on quality of life (QoL) in patients with moderate-to-severe, uncontrolled asthma.
Recent Findings
Tezepelumab improves pulmonary function tests (PFTs) and reduces the annualized asthma exacerbation rate (AAER) in patients with moderate-to-severe, uncontrolled asthma.
Summary
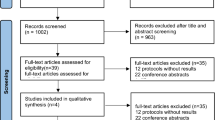
We searched MEDLINE, Embase, and Cochrane Library from inception to September 2022. We included randomized controlled trials comparing tezepelumab versus placebo in patients aged ≥ 12 years with asthma on medium- or high-dose inhaled corticosteroids with ≥ 1 additional controller medication for ≥ 6 months and who had ≥ 1 asthma exacerbation in the 12 months before enrollment. We estimated effects measures with a random-effects model. Of 239 records identified, three studies were included, with a total of 1,484 patients. Tezepelumab significantly decreased biomarkers of T helper 2-driven inflammation, including blood eosinophil count (MD -135.8 [95% CI -164.37, -107.23]) and fractional exhaled nitric oxide (MD -9.64 [95% CI -13.75, -5.53]); improved PFTs, including pre-bronchodilator forced expiratory volume in 1 s (MD 0.18 [95% CI 0.08–0.27]); reduced the AAER (MD 0.47 [95% CI 0.39–0.56]); improved asthma-specific health-related QoL in the Asthma Control Questionnaire-6 (MD -0.33 [95% CI -0.34, -0.32]), Asthma Quality of Life Questionnaire for 12 Years and Older (MD 0.34 [95% CI 0.33, -0.35]), Asthma Symptom Diary (MD -0.11 [95% CI -0.18, -0.04]), and European Quality of Life 5 Dimensions 5 Levels Questionnaire (SMD 3.29 [95% CI 2.03, 4.55]) scores, although not clinically important; and did not change key safety outcomes, including any adverse event (OR 0.78 [95% CI 0.56–1.09]).



Similar content being viewed by others
Data Availability
All data relevant to the study are included in the article or uploaded as supplementary information.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Published 2022. Accessed 18 Nov 2022. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
• Edris A, De Feyter S, Maes T, Joos G, Lahousse L. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respir Res. 2019;20(1):179. https://doi.org/10.1186/s12931-019-1138-3. This article evaluates new biologic agents for persistently uncontrolled asthma and compares their effects on asthma exacerbation rate.
•• Pelaia C, Pelaia G, Crimi C, et al. Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. Int J Mol Sci. 2021;22(9). https://doi.org/10.3390/ijms22094369. This article reviews the role of thymic stromal lymphopoietin in the pathogenesis of asthma and the rationale for the use of tezepelumab as an add-on therapy for severe, uncontrolled asthma.
Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–92. https://doi.org/10.1080/14728222.2020.1783242.
Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma (PATHWAY). N Engl J Med. 2017;377(10):936–46. https://doi.org/10.1056/NEJMoa1704064.
Rich HE, Antos D, Melton NR, Alcorn JF, Manni ML. Insights Into Type I and III Interferons in Asthma and Exacerbations. Front Immunol. 2020;11:574027. https://doi.org/10.3389/fimmu.2020.574027.
Corren J, Garcia Gil E, Griffiths JM, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021;126(2):187–93. https://doi.org/10.1016/j.anai.2020.10.008.
Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019;28(11):931–40. https://doi.org/10.1080/13543784.2019.1672657.
Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma (NAVIGATOR). N Engl J Med. 2021;384(19):1800–9. https://doi.org/10.1056/NEJMoa2034975.
Feist J, Lipari M, Kale-Pradhan P. Tezepelumab in the Treatment of Uncontrolled Severe Asthma. Ann Pharmacother. 2023;57(1):62–70. https://doi.org/10.1177/10600280221095540.
Matera MG, Rogliani P, Calzetta L, Cazzola M. TSLP Inhibitors for Asthma: Current Status and Future Prospects. Drugs. 2020;80(5):449–58. https://doi.org/10.1007/s40265-020-01273-4.
Wechsler ME, Menzies-Gow A, Brightling CE, et al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med. 2022;10(7):650–60. https://doi.org/10.1016/S2213-2600(21)00537-3.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editors. Cochrane handbook for systematic reviews of interventions version 6.3. (Updated February 2022). Cochrane; 2022.
Page M, Moher D, Bossuyt P, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372(160). https://doi.org/10.1136/bmj.n160.
Chagas G, Xavier D, Gomes L, Ferri-Guerra J, Oquet R. The Effects of Tezepelumab on The Quality of Life of Patients with Moderate-to-Severe, Uncontrolled Asthma: A Systematic Review and Meta-Analysis. PROSPERO. Published 2022. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=361442.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557 LP-560. https://doi.org/10.1136/bmj.327.7414.557.
Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. (The GRADE Working Group, ed.). 2013. https://guidelinedevelopment.org/handbook.
Bonini M, Di Paolo M, Bagnasco D, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev Off J Eur Respir Soc. 2020;29(156). https://doi.org/10.1183/16000617.0137-2019.
Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81–7. https://doi.org/10.1016/0895-4356(94)90036-1.
Globe G, Wiklund I, Mattera M, Zhang H, Revicki DA. Evaluating minimal important differences and responder definitions for the asthma symptom diary in patients with moderate to severe asthma. J Patient-Rep Outcomes. 2019;3(1):22. https://doi.org/10.1186/s41687-019-0109-2.
Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–24. https://doi.org/10.1081/copd-200053377.
Oppenheimer J, Hoyte FCL, Phipatanakul W, Silver J, Howarth P, Lugogo NL. Allergic and eosinophilic asthma in the era of biomarkers and biologics: similarities, differences and misconceptions. Ann Allergy Asthma Immunol. 2022;129(2):169–80. https://doi.org/10.1016/j.anai.2022.02.021.
Frey U, Brodbeck T, Majumdar A, et al. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature. 2005;438(7068):667–70. https://doi.org/10.1038/nature04176.
Menzies-Gow A, Steenkamp J, Singh S, et al. Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison. J Med Econ. 2022;25(1):679–90. https://doi.org/10.1080/13696998.2022.2074195.
Pitre T, Jassal T, Angjeli A, et al. A comparison of the effectiveness of biologic therapies for asthma: a systematic review and network meta-analysis. Ann Allergy Asthma Immunol Off Publ Am Coll. Published online December 2022. https://doi.org/10.1016/j.anai.2022.12.018.
Nopsopon T, Lassiter G, Chen M-L, et al. Comparative Efficacy of Tezepelumab to Mepolizumab, Benralizumab, and Dupilumab in Eosinophilic Asthma: A Bayesian Network Meta-analysis. J Allergy Clin Immunol Published online. 2022. https://doi.org/10.1016/j.jaci.2022.11.021.
Shaban Abdelgalil M, Ahmed Elrashedy A, Awad AK, et al. Safety and efficacy of tezepelumab vs. placebo in adult patients with severe uncontrolled asthma: a systematic review and meta-analysis. Sci Rep. 2022;12(1):20905. https://doi.org/10.1038/s41598-022-24763-9.
Zoumot Z, Al Busaidi N, Tashkandi W, et al. Tezepelumab for Patients with Severe Uncontrolled Asthma: A Systematic Review and Meta-Analysis. J Asthma Allergy. 2022;15:1665–79. https://doi.org/10.2147/JAA.S378062.
Ando K, Fukuda Y, Tanaka A, Sagara H. Comparative Efficacy and Safety of Tezepelumab and Other Biologics in Patients with Inadequately Controlled Asthma According to Thresholds of Type 2 Inflammatory Biomarkers: A Systematic Review and Network Meta-Analysis. Cells. 2022;11(5). https://doi.org/10.3390/cells11050819.
U.S. Food and Drug Administration. FDA approves maintenance treatment for severe asthma. Published 2021. Accessed 1 Jan 2023. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma.
Tay TR, Pham J, Hew M. Addressing the impact of ethnicity on asthma care. Curr Opin Allergy Clin Immunol. 2020;20(3):274–81. https://doi.org/10.1097/ACI.0000000000000609.
Acknowledgements
The authors would like to thank Rhanderson Cardoso, MD, MHS (Brigham and Women's Hospital, Harvard Medical School) for his valuable support in conducting this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the search strategy and selection and data extraction criteria. GC performed the selection of studies, data extraction, methodological quality assessment, and certainty assessment, provided statistical expertise, wrote, and published the protocol, and drafted the manuscript. DX performed the selection of studies, data extraction, methodological quality assessment, and certainty assessment, provided statistical expertise, and drafted the manuscript. LG performed the selection of studies and data extraction, provided statistical expertise, and drafted the manuscript. JFG performed the methodological quality and certainty assessment and drafted the manuscript. RHO contributed to the critical revision of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have declared no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chagas, G.C.L., Xavier, D., Gomes, L. et al. Effects of Tezepelumab on Quality of Life of Patients with Moderate-to-Severe, Uncontrolled Asthma: Systematic Review and Meta-Analysis. Curr Allergy Asthma Rep 23, 287–298 (2023). https://doi.org/10.1007/s11882-023-01085-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-023-01085-y