Abstract
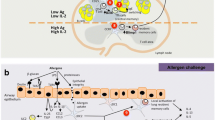
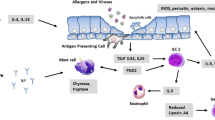
Genetic predisposition and environmental instructions tune thresholds for activation of T cells, other inflammatory cells, and resident tissue cells in asthmatic inflammation. Selective migration of peripheral-blood T cells to the lungs, their survival and reactivation in the submucosa, and their effector functions represent sequential immunologic events. Activation-induced T-cell death and peripheral T-cell tolerance are critical events in disease pathogenesis. As a mechanism for peripheral Th2 response in atopic diseases, particularly, the high interferon (IFN)-γ-producing Th1 compartment of activated effector T cells shows increased activation-induced cell death, skewing the immune response toward surviving Th2 cells in allergic asthma. After migration to asthmatic lung, these cells switch on effector cytokines and induce bronchial epithelial apoptosis with mainly IFN-γ, tumor necrosis factor (TNF)-α, and Fas-ligand. In addition, skewing of allergen-specific effector T cells to T-regulatory cells appears to be an essential event in the control of harmful immune response induced by allergens as a possible means for remedy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References and Recommended Reading
Akdis CA, Blaser K, Akdis M: Apoptosis in tissue inflammation and allergic disease. Curr Opin Immunol 2004, 16:717–723.
Akdis M, Trautmann A, Klunker S, et al.: T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J 2003, 17:1026–1035.
Simon H-U, Blaser K: Inhibition of programmed eosinophil death: a key pathogenic event for eosinophilia. Immunol Today 1995, 16:53–55.
Trautmann A, Akdis M, Kleemann D, et al.: T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000, 106:25–35.
Trautmann A, Schmid-Grendelmeier P, Krüger K, et al.: T cells and eosinophils cooperate in the induction of bronchial epithelial apoptosis in asthma. J Allergy Clin Immunol 2002, 109:329–337.
Trautmann A, Kruger K, Akdis M, et al.: Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol 2005, 138:142–150.
Romagnani S: Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol 2004, 113:395–400.
Yssel H, Groux H: Characterization of T cell subpopulations involved in the pathogenesis of asthma and allergic diseases. Int Arch Allergy Immunol 2000, 121:10–18.
Jutel M, Akdis M, Budak F, et al.: IL-10 and TGF-beta cooperate in regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003, 33:1205–1214. This study demonstrates that immunotherapy that is specific for the house dust mite allergen induces an antigen-specific suppressive activity in the CD4+CD25+ T cells of allergic individuals. A deviation toward a regulatory or suppressor T cell response during specific immunotherapy and in normal immunity is shown to be a key event in the healthy immune response to mucosal antigens.
Akdis CA, Blaser K: IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J 1999, 13:603–609.
Akdis CA, Blesken T, Akdis M, et al.: Role of IL-10 in specific immunotherapy. J Clin Invest 1998, 102:98–106.
Akdis M, Verhagen J, Taylor A, et al.: Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med 2004, 199:1567–1575. Allergen-specific CD4+ T cells producing IFN-γ, IL-4, and IL-10 show characteristics of Th1-, Th2-, and T regulatory 1 (Tr1)-like cells, respectively. Tr1 cells consistently represent the dominant subset specific for common environmental allergens in healthy individuals; by contrast, there is a high frequency of allergenspeci fic IL-4-secreting T cells in allergic individuals. Tr1 cells use several mechanisms of suppression, including IL-10 and TGF-β as secreted cytokines, and CTLA-4 and PD-1 as surface molecules.
Chen Y, Kuchroo VK, Inobe J, et al.: Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 1994, 265:1237–1240.
Powrie F, Correa-Oliveira R, Mauze S, Coffman RL: Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med 1994, 179:589–600.
Groux H, O’Garra A, Bigler M, et al.: A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389:737–742.
Taams LS, Smith J, Rustin MH, et al.: Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol 2001, 31:1122–1131.
Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH: Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 2000, 192:1213–1222.
Cottrez F, Hurst SD, Coffman RL, Groux H: T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol 2000, 165:4848–4853.
Finotto S, Neurath MF, Glickman JN, et al.: Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 2002, 295:336–338.
Skapenko A, Leipe J, Niesner U, et al.: GATA-3 in human T cell Helper type 2 development. J Exp Med 2004, 199:423–428.
Amsen D, Blander JM, Lee GR, et al.: Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 2004, 117:515–526. This study demonstrates that antigen-presenting cells (APCs) use the notch pathway to instruct Th cell differentiation. The expression of different notch ligands on APCs is induced by Th1-or Th2-promoting stimuli. Delta induces Th1, whereas Jagged constitutes an instructive signal for Th2 differentiation that is independent of IL-4 and STAT-6.
Picker LJ, Michie SA, Rott LS, Butcher EC: A unique phenotype of skin associated lymphocytes in humans: preferential expression of the HECA-452 epitope by benign and malignant T-cells at cutaneous sites. Am J Pathol 1990, 136:1053–1061.
Santamaria Babi LF, Picker LJ, Perez Soler MT, et al.: Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med 1995, 181:1935–1940.
Akdis M, Akdis CA, Weigl L, et al.: Skin-homing, CLA+ memory T cells are activated in atopic dermatitis and regulate IgE by an IL-13-dominated cytokine pattern. IgG4 counter-regulation by CLA-memory T cells. J Immunol 1997, 159:4611–4619.
Akdis M, Simon H-U, Weigl L, et al.: Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol 1999, 163:466–475.
Liu Y, Janeway CA Jr: Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med 1990, 172:1735–1739.
Wu CY, Kirman JR, Rotte MJ, et al.: Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol 2002, 3:852–858.
Zhang X, Brunner T, Carter L, et al.: Unequal death in T helper cell (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med 1997, 185:1837–1849.
Ledru E, Lecoeur H, Garcia S, et al.: Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol 1998, 160:3194–3206.
Varadhachary AS, Peter ME, Perdow SN, et al.: Selective up-regulation of phosphotidyinositol 3’-kinase activity in Th2 cells inhibits caspase-8 cleavage at the deathinducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol 1999, 163:4772–4779.
Trautmann A, Altznauer F, Akdis M, et al.: The differential fate of cadherins during T cell-induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Derm 2001, 117:927–934.
Giancotti FG, Rouslahti E: Integrin signaling. Science 1999, 285:1028–1032.
Gershon RK, Kondo K: Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970, 18:723–737.
Lee WY, Sehon AH: Abrogation of reaginic antibodies with modified proteins. Nature 1977, 267:618–620.
Rocklin RE, Sheffer A, Greineder DK, Melmon KL: Generation of antigen-specific suppressor cells during allergy desensitization. N Engl J Med 1980, 302:1213–1219.
Green DR, Webb DR: Saying the ‘S’ word in public. Immunol Today 1993, 14:523–525.
Levings MK, Sangregorio R, Galbiati F, et al.: IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol 2001, 166:5530–5539.
Akdis CA, Akdis M, Blesken T, et al.: Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest 1996, 98:1676–1683.
Müller UR, Akdis CA, Fricker M, et al.: Successful immunotherapy with T cell epitope peptides of bee venom phospholipase A2 induces specific T cell anergy in bee sting allergic patients. J Allergy Clin Immunol 1998, 101:747–754.
Oldfield WL, Larche M, Kay AB: Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet 2002, 360:47–53.
Kussebi F, Karamloo F, Rhyner C, et al.: A major allergen gene-fusion protein for potential usage in allergenspeci fic immunotherapy. J Allergy Clin Immunol 2005, 115:323–329. This fusion protein is composed of the two major bee venom allergens: phospholipase A2 (Api m 1) and hyaluronidase (Api m 2). The Api m [1/2] fusion protein bypasses IgE binding and mast cell/basophil IgE FcɛRI cross-linking and protects from IgE development in mice.
Karamloo F, Schmid-Grendelmeier P, Kussebi F, et al.: Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol 2005, 35:3268–3276. This is the first study showing a chimeric vaccine of three major bee venom allergens. The Api m [1/2/3] is composed of six different fragments of three different allergens expressed as a single protein. Api m [1/2/3] does not express B-cell epitopes and does not bind IgE, but preserves the entire T-cell epitope. The Api m [1/2/3] vaccine does not induce skin-test reactivity in bee venom-allergic humans and shows almost abolished IgE binding. Treatment of mice with Api m [1/2/3] led to a significant reduction of specific IgE development toward native allergens representing a protective vaccine effect in vivo.
Punnonen J, De Waal Malefyt R, Van Vlasselaer P, et al.: IL-10 and viral IL-10 prevent IL-4-induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol 1993, 151:1280–1289.
Sonoda E, Matsumoto R, Hitoshi Y, et al.: Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med 1989, 170:1415–1420.
Walker C, Virchow J-C, Bruijnzeel PLB, Blaser K: T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol 1991, 146:1829–1835.
Schleimer RP, Derse CP, Friedman B, et al.: Regulation of human basophil mediator release by cytokines. I. Interaction with anti-inflammatory steroids. J Immunol 1989, 143:1310–1327.
Treter S, Luqman M: Antigen-specific T cell tolerance downregulates mast cell responses in vivo. Cell Immunol 2000, 206:116–124.
Shim YK, Kim BS, Cho SH, et al.: Allergen-specific conventional immunotherapy decreases immunoglobulin E-mediated basophil histamine releasability. Clin Exp Allergy 2003, 33:52–57.
Marshall JS, Leal-Berumen I, Nielsen L, et al.: Interleukin (IL)-10 inhibits long-term IL-6 production, but not preformed mediator release from rat peritoneal mast cells. J Clin Invest 1996, 97:1122–1128.
Schandane L, Alonso-Vega C, Willems F, et al.: B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol 1994, 152:4368–4374.
Ohkawara Y, Lim KG, Glibetic M, et al.: CD40 expression by human peripheral blood eosinophils. J Clin Invest 1996, 97:1761–1766.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meiler, F., Zimmermann, M., Blaser, K. et al. T-cell subsets in the pathogenesis of human asthma. Curr Allergy Asthma Rep 6, 91–96 (2006). https://doi.org/10.1007/s11882-006-0045-0
Issue Date:
DOI: https://doi.org/10.1007/s11882-006-0045-0