Opinion Statement
Gastric cancer (GC) is a deadly disease worldwide, and trastuzumab in combination with chemotherapy has been the standard first-line treatment for HER2-positive GC following the TOGA trial. Besides adjuvant therapy, HER2-directed therapy is widely used as neoadjuvant or translational therapy, and survival benefit even surgical opportunities is seen in these patients. However, resistance is not rare in recent years, and the second-line treatment for trastuzumab beyond progression has received widespread attention in GC. Moreover, current evidence cannot recommend trastuzumab for patients with IHC1+ HER2 low expression GC yet. Researchers are currently investigating whether GC patients with low HER2 expression could also benefit from HER2-directed therapies. In addition to using HER2 as a target for targeted therapy, HER2-mediated targeted delivery of cytotoxic drugs and targeted immunity have made important contributions to overcoming trastuzumab resistance in recent trials. HER2/neu-derived peptide epitopes vaccination and HER2-specific chimeric antigen receptor (CAR) therapy focus on reestablishing anti-tumor immunity in different ways and show significant anti-tumor activity. Other antibodies that target different regions of the HER2 receptor or block key downstream pathways such as AKT or PI3K also offer potential anti-tumor activity against HER2. HER2 use in GC will not be hampered by resistance or low expression and will play a bigger role. We review the current efforts to enable GC patients with trastuzumab-resistant and HER2 low-expressing accessible to HER2 targeted therapy and present our consideration for future HER2 in GC.



Similar content being viewed by others
Data Availability
All data supporting this study have been presented in the full text. Other relevant materials can be obtained on reasonable request to the corresponding author.
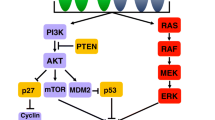
Figures and tables used in this article are original and have not been published previously.
References and Recommended Reading
Smyth EC, et al. Gastric cancer. Lancet. 2020;396(10251):635–48. https://doi.org/10.1016/s0140-6736(20)31288-5.
Guan WL, et al. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16(1):57. https://doi.org/10.1186/s13045-023-01451-3.
Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. https://doi.org/10.1016/s0140-6736(10)61121-x.
Ajani JA, et al. Gastric Cancer, Version 22022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(2):167–92. https://doi.org/10.6004/jnccn.2022.0008.
Blangé D, et al. Resistance mechanisms to HER2-targeted therapy in gastroesophageal adenocarcinoma: A systematic review. Cancer Treat Rev. 2022;108:102418. https://doi.org/10.1016/j.ctrv.2022.102418.
Shitara K, et al. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: A subgroup analysis of the GATSBY study. Asia Pac J Clin Oncol. 2019;16(1):5–13. https://doi.org/10.1111/ajco.13243.
Ruschoff J, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. https://doi.org/10.1038/modpathol.2011.198.
Tokunaga M, et al. Early endpoints of a randomized phase II trial of preoperative chemotherapy with S-1/CDDP with or without trastuzumab followed by surgery for HER2-positive resectable gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301C (Trigger Study). Gastric Cancer. 2024;27(3):580–9. https://doi.org/10.1007/s10120-024-01467-9.
Hofheinz RD, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149(6):1322–31. https://doi.org/10.1002/ijc.33696.
Rivera F, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67. https://doi.org/10.1016/j.ejca.2020.12.005.
Janjigian YY, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31. https://doi.org/10.1016/s1470-2045(20)30169-8.
Janjigian YY, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30. https://doi.org/10.1038/s41586-021-04161-3.
Janjigian YY, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402(10418):2197–208. https://doi.org/10.1016/s0140-6736(23)02033-0.
Lee CK, et al. A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer. Nat Commun. 2022;13(1):6002. https://doi.org/10.1038/s41467-022-33267-z.
Montagnani F, et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14(1):50–5. https://doi.org/10.1007/s10120-011-0007-7.
Kim GM, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48(4):518–26. https://doi.org/10.1016/j.ejca.2011.12.017.
Mori Y, et al. Phase II Prospective Study of Trastuzumab in Combination with S-1 and Oxaliplatin (SOX100) Therapy for HER2-Positive Advanced Gastric Cancer. J Gastrointest Cancer. 2022;53(4):930–8. https://doi.org/10.1007/s12029-021-00711-0.
Takahari D, et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22(6):1238–46. https://doi.org/10.1007/s10120-019-00973-5.
Yuki S, et al. Multicenter phase II study of SOX plus trastuzumab for patients with HER2(+) metastatic or recurrent gastric cancer: KSCC/HGCSG/CCOG/PerSeUS 1501B. Cancer Chemother Pharmacol. 2020;85(1):217–23. https://doi.org/10.1007/s00280-019-03991-3.
Makiyama A, et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J Clin Oncol. 2020;38(17):1919–27. https://doi.org/10.1200/jco.19.03077.
Horita Y, et al. Phase II clinical trial of second-line weekly paclitaxel plus trastuzumab for patients with HER2-positive metastatic gastric cancer. Anticancer Drugs. 2019;30(1):98–104. https://doi.org/10.1097/cad.0000000000000691.
Kawamoto Y, et al. Phase II Study of Continued Trastuzumab Plus Irinotecan in Patients with HER2-positive Gastric Cancer Previously Treated with Trastuzumab (HGCSG 1201). Oncologist. 2022;27(5):340-e374. https://doi.org/10.1093/oncolo/oyab062.
Takahashi M, et al. Phase II Study of the Reuse of Trastuzumab with Docetaxel beyond Progression after First-Line Treatment in Second-Line Treatment for Unresectable, Metastatic Gastric Cancer (T-CORE1203). Tohoku J Exp Med. 2021;254(1):49–55. https://doi.org/10.1620/tjem.254.49.
Sun J, et al. From Anti-HER-2 to Anti-HER-2-CAR-T Cells: An Evolutionary Immunotherapy Approach for Gastric Cancer. J Inflamm Res. 2022;15:4061–85. https://doi.org/10.2147/jir.S368138.
Zhang H, et al. Anti-HER2 scFv-CCL19-IL7 recombinant protein inhibited gastric tumor growth in vivo. Sci Rep. 2022;12(1):10461. https://doi.org/10.1038/s41598-022-14336-1.
Song Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9(10):867–78. https://doi.org/10.1007/s13238-017-0384-8.
Luo F, et al. Bifunctional αHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer. Cell Res. 2016;26(7):850–3. https://doi.org/10.1038/cr.2016.81.
Dong X, et al. Efficacy evaluation of chimeric antigen receptor-modified human peritoneal macrophages in the treatment of gastric cancer. Br J Cancer. 2023;129(3):551–62. https://doi.org/10.1038/s41416-023-02319-6.
Sheikhlary S, et al. Recent Findings on Therapeutic Cancer Vaccines: An Updated Review. Biomolecules. 2024;14(4). https://doi.org/10.3390/biom14040503.
Wiedermann U, et al. Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/neu-Overexpressing Advanced Gastric Cancer-Results from Phase Ib Trial IMU.ACS.001. Clin Cancer Res. 2021;27(13):3649-3660. https://doi.org/10.1158/1078-0432.Ccr-20-3742.
Ede NJ, et al. Development of the B cell cancer vaccine HER-vaxx for the treatment of her-2 expressing cancers. Front Oncol. 2022;12:939356. https://doi.org/10.3389/fonc.2022.939356.
Hu HH, et al. HER2(+) advanced gastric cancer: Current state and opportunities (Review). Int J Oncol. 2024;64(4). https://doi.org/10.3892/ijo.2024.5624.
Jung M, et al. First-in-Human Phase 1 Study of a B Cell- and Monocyte-Based Immunotherapeutic Vaccine against HER2-Positive Advanced Gastric Cancer. Cancer Res Treat. 2024;56(1):208–18. https://doi.org/10.4143/crt.2022.1328.
Wiedermann U, et al. A phase Ib/II open label study of IMU-131 HER2/Neu peptide vaccine plus cisplatin and either 5-fluorouracil or capecitabine chemotherapy in patients with HER2/Neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction. 2019;37(4_suppl):p. TPS176-TPS176. https://doi.org/10.1200/JCO.2019.37.4_suppl.TPS176.
Metz H, et al. SBT6050, a HER2-directed TLR8 therapeutic, as a systemically administered, tumor-targeted human myeloid cell agonist. 2020;38(15_suppl):3110-3110. https://doi.org/10.1200/JCO.2020.38.15_suppl.3110.
Li BT, et al. A phase 1/2 study of a first-in-human immune-stimulating antibody conjugate (ISAC) BDC-1001 in patients with advanced HER2-expressing solid tumors. 2023;41(16_suppl):2538-2538. https://doi.org/10.1200/JCO.2023.41.16_suppl.2538.
Shen A, et al. A novel 4–1BB/HER2 bispecific antibody shows potent antitumor activities by increasing and activating tumor-infiltrating T cells. Am J Cancer Res. 2023;13(7):3246–56.
Sun W, et al. CD40×HER2 bispecific antibody overcomes the CCL2-induced trastuzumab resistance in HER2-positive gastric cancer. J Immunother Cancer. 2022;10(7). https://doi.org/10.1136/jitc-2022-005063.
Tateyama N, et al. Antitumor Activity of an Anti-EGFR/HER2 Bispecific Antibody in a Mouse Xenograft Model of Canine Osteosarcoma. Pharmaceutics. 2022;14(11). https://doi.org/10.3390/pharmaceutics14112494.
Oberg HH, et al. Tribody [(HER2)(2)xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front Immunol. 2018;9:814. https://doi.org/10.3389/fimmu.2018.00814.
Yu S, et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019;38(1):355. https://doi.org/10.1186/s13046-019-1354-1.
Lin W, et al. Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis. Adv Sci (Weinh). 2023;10(30):e2303908. https://doi.org/10.1002/advs.202303908.
Chau CH, et al. Antibody-drug conjugates for cancer. Lancet. 2019;394(10200):793–804. https://doi.org/10.1016/s0140-6736(19)31774-x.
Shin SH, et al. An Elaborate New Linker System Significantly Enhances the Efficacy of an HER2-Antibody-Drug Conjugate against Refractory HER2-Positive Cancers. Adv Sci (Weinh). 2021;8(23):e2102414. https://doi.org/10.1002/advs.202102414.
Lordick F, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20. https://doi.org/10.1016/j.annonc.2022.07.004.
Van Cutsem E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24(7):744–56. https://doi.org/10.1016/s1470-2045(23)00215-2.
Shitara K, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382(25):2419–30. https://doi.org/10.1056/NEJMoa2004413.
Meric-Bernstam F, et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024;42(1):47–58. https://doi.org/10.1200/jco.23.02005.
Lewis Phillips GD, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. https://doi.org/10.1158/0008-5472.Can-08-1776.
Thuss-Patience PC, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53. https://doi.org/10.1016/s1470-2045(17)30111-0.
Wang H, et al. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in human epidermal growth factor receptor 2-positive gastric cancer cells. Cancer Sci. 2017;108(7):1458–68. https://doi.org/10.1111/cas.13253.
Deeks ED. Disitamab Vedotin: First Approval. Drugs. 2021;81(16):1929–35. https://doi.org/10.1007/s40265-021-01614-x.
Peng Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41(11):1173–82. https://doi.org/10.1002/cac2.12214.
Zong HF, et al. A novel bispecific antibody drug conjugate targeting HER2 and HER3 with potent therapeutic efficacy against breast cancer. Acta Pharmacol Sin. 2024. https://doi.org/10.1038/s41401-024-01279-8.
Meric-Bernstam F, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022;23(12):1558–70. https://doi.org/10.1016/s1470-2045(22)00621-0.
DiPeri TP, et al. Co-clinical Trial of Novel Bispecific Anti-HER2 Antibody Zanidatamab in Patient-Derived Xenografts. Cancer Discov. 2024;14(5):828–45. https://doi.org/10.1158/2159-8290.Cd-23-0838.
Harding JJ, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772–82. https://doi.org/10.1016/s1470-2045(23)00242-5.
Xu J, et al. KN026 (anti-HER2 bispecific antibody) in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancer. Eur J Cancer. 2023;178:1–12. https://doi.org/10.1016/j.ejca.2022.10.004.
Huang S, et al. Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody. MAbs. 2018;10(6):864-875. https://doi.org/10.1080/19420862.2018.1486946.
Tabernero J, et al. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18(29):3255–66. https://doi.org/10.2217/fon-2022-0595.
Kaplon H, et al. Antibodies to watch in 2023. MAbs. 2023;15(1):2153410. https://doi.org/10.1080/19420862.2022.2153410.
Haense N, et al. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016;16:420. https://doi.org/10.1186/s12885-016-2449-0.
Jäger M, et al. The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res. 2009;69(10):4270–6. https://doi.org/10.1158/0008-5472.Can-08-2861.
Pegram MD, et al. First-in-Human, Phase 1 Dose-Escalation Study of Biparatopic Anti-HER2 Antibody-Drug Conjugate MEDI4276 in Patients with HER2-positive Advanced Breast or Gastric Cancer. Mol Cancer Ther. 2021;20(8):1442–53. https://doi.org/10.1158/1535-7163.Mct-20-0014.
Park JJ, et al. Safety and efficacy of JSKN003 in patients with advanced/metastatic solid tumors: A first-in-human, dose-escalation, multicenter, open-label, phase I study. 2024;42(16_suppl):3038-3038. https://doi.org/10.1200/JCO.2024.42.16_suppl.3038.
Gu Y, et al. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharm Sin B. 2024;14(5):1965–86. https://doi.org/10.1016/j.apsb.2024.01.009.
Zhuang W, et al. Generation of a Novel SORT1×HER2 Bispecific Antibody-Drug Conjugate Targeting HER2-Low-Expression Tumor. Int J Mol Sci. 2023;24(22). https://doi.org/10.3390/ijms242216056.
Pivot X, et al. Milestones over the development of SB3, a trastuzumab biosimilar. Future Oncol. 2018;14(27):2795–803. https://doi.org/10.2217/fon-2018-0270.
Paik J. PF-05280014: A Trastuzumab Biosimilar. BioDrugs. 2018;32(5):515–8. https://doi.org/10.1007/s40259-018-0308-z.
Dhillon S. ABP 980: A Trastuzumab Biosimilar. BioDrugs. 2018;32(5):511–4. https://doi.org/10.1007/s40259-018-0305-2.
Fu C, et al. Clinical development of CT-P6 in HER2 positive breast cancer. Expert Opin Biol Ther. 2019;19(10):987–92. https://doi.org/10.1080/14712598.2019.1665019.
Audran R, et al. Immunomodulation profile of the biosimilar trastuzumab MYL-1401O in a bioequivalence phase I study. Sci Rep. 2024;14(1):12872. https://doi.org/10.1038/s41598-024-61265-2.
Miller EM, et al. Biosimilars for breast cancer: a review of HER2-targeted antibodies in the United States. Ther Adv Med Oncol. 2019;11:1758835919887044. https://doi.org/10.1177/1758835919887044.
Allahyari A, et al. Comparing efficacy and safety of P013, a proposed pertuzumab biosimilar, with the reference product in HER2-positive breast cancer patients: a randomized, phase III, equivalency clinical trial. BMC Cancer. 2022;22(1):960. https://doi.org/10.1186/s12885-022-09895-5.
Yang J, et al. HLX11, a Proposed Pertuzumab Biosimilar: Pharmacokinetics, Immunogenicity, and Safety Profiles Compared to Three Reference Biologic Products (US-, EU-, and CN-Approved Pertuzumab) Administered to Healthy Male Subjects. BioDrugs. 2022;36(3):393–409. https://doi.org/10.1007/s40259-022-00534-w.
Bang YJ, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61. https://doi.org/10.1093/annonc/mdx002.
Markham A. Margetuximab: First Approval. Drugs. 2021;81(5):599–604. https://doi.org/10.1007/s40265-021-01485-2.
Catenacci DVT, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7(5):100563. https://doi.org/10.1016/j.esmoop.2022.100563.
Catenacci DVT, et al. Antitumor activity of margetuximab (M) plus pembrolizumab (P) in patients (pts) with advanced HER2+ (IHC3+) gastric carcinoma (GC). 2019;37(4_suppl):65-65. https://doi.org/10.1200/JCO.2019.37.4_suppl.65.
von Minckwitz G, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122–31. https://doi.org/10.1056/NEJMoa1703643.
Tabernero J, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84. https://doi.org/10.1016/s1470-2045(18)30481-9.
Liu T, et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond). 2019;39(1):38. https://doi.org/10.1186/s40880-019-0384-6.
Tabernero J, et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. Gastric Cancer. 2023;26(1):123–31. https://doi.org/10.1007/s10120-022-01335-4.
Wagner AD, et al. EORTC-1203 GITC "INNOVATION": Integration of trastuzumab (T), with or without pertuzumab (P), into perioperative chemotherapy of HER-2 positive stomach cancer: Overall survival results. 2025;43(4_suppl):LBA331-LBA331. https://doi.org/10.1200/JCO.2025.43.4_suppl.LBA331.
Hofheinz RD, et al. FLOT Versus FLOT/Trastuzumab/Pertuzumab Perioperative Therapy of Human Epidermal Growth Factor Receptor 2-Positive Resectable Esophagogastric Adenocarcinoma: A Randomized Phase II Trial of the AIO EGA Study Group. J Clin Oncol. 2022;40(32):3750–61. https://doi.org/10.1200/jco.22.00380.
Zhu X, et al. HLX22, an anti-HER-2 monoclonal antibody, in patients with advanced solid tumors overexpressing human epidermal growth factor receptor 2: an open-label, dose-escalation, phase 1 trial. Invest New Drugs. 2023;41(3):473–82. https://doi.org/10.1007/s10637-023-01338-7.
Wei R, et al. Dual targeting non-overlapping epitopes in HER2 domain IV substantially enhanced HER2/HER2 homodimers and HER2/EGFR heterodimers internalization leading to potent antitumor activity in HER2-positive human gastric cancer. J Transl Med. 2024;22(1):641. https://doi.org/10.1186/s12967-024-05453-8.
Pereira PMR, et al. Caveolin-1 temporal modulation enhances antibody drug efficacy in heterogeneous gastric cancer. Nat Commun. 2022;13(1):2526. https://doi.org/10.1038/s41467-022-30142-9.
Pereira PMR, et al. Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat Commun. 2018;9(1):5137. https://doi.org/10.1038/s41467-018-07608-w.
Rao Y, et al. Statins enhance the efficacy of HER2-targeting radioligand therapy in drug-resistant gastric cancers. Proc Natl Acad Sci U S A. 2023;120(14):e2220413120. https://doi.org/10.1073/pnas.2220413120.
Wang J, et al. Disrupting Circadian Rhythm via the PER1-HK2 Axis Reverses Trastuzumab Resistance in Gastric Cancer. Cancer Res. 2022;82(8):1503–17. https://doi.org/10.1158/0008-5472.Can-21-1820.
Wang S, et al. Circadian rhythm as a key player in cancer progression as well as a therapeutic target in HER2-positive advanced gastric cancer treatment. Front Oncol. 2023;13:1240676. https://doi.org/10.3389/fonc.2023.1240676.
Rocca A, et al. Phase II study of liposomal doxorubicin, docetaxel and trastuzumab in combination with metformin as neoadjuvant therapy for HER2-positive breast cancer. Ther Adv Med Oncol. 2021;13:1758835920985632. https://doi.org/10.1177/1758835920985632.
Kolbe K, et al. Deviating HER2 test results in gastric cancer: analysis from the prospective multicenter VARIANZ study. J Cancer Res Clin Oncol. 2023;149(3):1319–29. https://doi.org/10.1007/s00432-022-04208-6.
Huang D, et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS ONE. 2013;8(11): e80290. https://doi.org/10.1371/journal.pone.0080290.
Grenda A, et al. HER2 gene assessment in liquid biopsy of gastric and esophagogastric junction cancer patients qualified for surgery. BMC Gastroenterol. 2020;20(1):382. https://doi.org/10.1186/s12876-020-01531-5.
Tominaga N, et al. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer. 2016;19(2):553–60. https://doi.org/10.1007/s10120-015-0502-3.
Gullo I, et al. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc Int Open. 2015;3(2):E165–70. https://doi.org/10.1055/s-0034-1391359.
Xu C, et al. Dual block HER2 assessment increased HER2 immunohistochemistry positive rate in resected specimens of gastric cancer: a prospective multicenter clinical trial from China. Diagn Pathol. 2022;17(1):54. https://doi.org/10.1186/s13000-022-01230-7.
Fan XS, et al. Differences in HER2 over-expression between proximal and distal gastric cancers in the Chinese population. World J Gastroenterol. 2013;19(21):3316–23. https://doi.org/10.3748/wjg.v19.i21.3316.
Pyo JS, et al. Concordance rate between HER2 immunohistochemistry and in situ hybridization in gastric carcinoma: systematic review and meta-analysis. Int J Biol Markers. 2016;31(1):e1-10. https://doi.org/10.5301/jbm.5000171.
Staněk L, et al. Comparison of immunohistochemistry, four in situ hybridization methods and quantitative polymerase chain reaction for the molecular diagnosis of HER2 status in gastric cancer: a study of 55 cases. Mol Med Rep. 2014;10(5):2669–74. https://doi.org/10.3892/mmr.2014.2530.
Yamashita-Kashima Y, et al. Importance of formalin fixing conditions for HER2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer. 2014;17(4):638–47. https://doi.org/10.1007/s10120-013-0329-8.
Gündisch S, et al. Critical roles of specimen type and temperature before and during fixation in the detection of phosphoproteins in breast cancer tissues. Lab Invest. 2015;95(5):561–71. https://doi.org/10.1038/labinvest.2015.37.
Ge X, et al. Clinical significance of assessing Her2/neu expression in gastric cancer with dual tumor tissue paraffin blocks. Hum Pathol. 2015;46(6):850–7. https://doi.org/10.1016/j.humpath.2015.02.011.
Hofmann M, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. https://doi.org/10.1111/j.1365-2559.2008.03028.x.
Radu OM, et al. HER2 amplification in gastroesophageal adenocarcinoma: correlation of two antibodies using gastric cancer scoring criteria, H score, and digital image analysis with fluorescence in situ hybridization. Am J Clin Pathol. 2012;137(4):583–94. https://doi.org/10.1309/ajcpxqvs6yghpdcy.
Tobias J, et al. Preclinical and Clinical Observations Implying Combination Therapy to Enhance the Efficacy of the Her-2/neu B-Cell Peptide-Based Vaccine HER-Vaxx and to Prevent Immune Evasion. Int J Mol Sci. 2023;25(1). https://doi.org/10.3390/ijms25010287.
Kasper S, et al. Targeted therapies in gastroesophageal cancer. Eur J Cancer. 2014;50(7):1247–58. https://doi.org/10.1016/j.ejca.2014.01.009.
Endo S, et al. Trastuzumab With S-1 Plus Cisplatin in HER2-positive Advanced Gastric Cancer Without Measurable Lesions: OGSG 1202. Anticancer Res. 2019;39(2):1059–65. https://doi.org/10.21873/anticanres.13213.
Rivera F, et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 2019;83(6):1175–81. https://doi.org/10.1007/s00280-019-03820-7.
Abali H, et al. A Phase II Study of the Combination of Oxaliplatin, Capecitabine, and Trastuzumab and Chemoradiotherapy in the Adjuvant Setting in Operated Patients With HER2-positive Gastric or Gastroesophageal Junction Cancer (TOXAG Study): A Turkish Oncology Group Study. Am J Clin Oncol. 2021;44(7):301–7. https://doi.org/10.1097/coc.0000000000000825.
Mondaca S, et al. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer. 2019;22(2):355–62. https://doi.org/10.1007/s10120-018-0861-7.
Pirrelli M, et al. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig Dis Sci. 2013;58(2):397–404. https://doi.org/10.1007/s10620-012-2357-3.
Watson S, et al. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study. Ann Oncol. 2013;24(12):3035–9. https://doi.org/10.1093/annonc/mdt393.
Yoshida H, et al. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch. 2014;465(2):145–54. https://doi.org/10.1007/s00428-014-1597-3.
Nergiz D, et al. Concordance of HER2 status tested by IHC and FISH in biopsy and surgical resection specimens and comparison with clinicopathological features in gastric carcinoma. Indian J Pathol Microbiol. 2022;65(2):321–7. https://doi.org/10.4103/ijpm.Ijpm_535_20.
Fazlollahi L, et al. HER2 Heterogeneity in Gastroesophageal Cancer Detected by Testing Biopsy and Resection Specimens. Arch Pathol Lab Med. 2018;142(4):516–22. https://doi.org/10.5858/arpa.2017-0039-OA.
Huang SC, et al. HER2 testing in paired biopsy and excision specimens of gastric cancer: the reliability of the scoring system and the clinicopathological factors relevant to discordance. Gastric Cancer. 2016;19(1):176–82. https://doi.org/10.1007/s10120-014-0453-0.
Lee S, et al. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59(5):832–40. https://doi.org/10.1111/j.1365-2559.2011.04017.x.
Grillo F, et al. The Reliability of Endoscopic Biopsies in Assessing HER2 Status in Gastric and Gastroesophageal Junction Cancer: A Study Comparing Biopsies with Surgical Samples. Transl Oncol. 2013;6(1):10–6. https://doi.org/10.1593/tlo.12334.
Qiu MZ, et al. Comparison of HER2 and Lauren Classification between Biopsy and Surgical Resection Samples, Primary and Metastatic Samples of Gastric Cancer. J Cancer. 2017;8(17):3531–7. https://doi.org/10.7150/jca.19984.
Funding
This research was supported by National Natural Science Foundation of China (82473266, 82160129), Key Project of Science and Technology in Gansu province (22ZD6FA054), Major scientific research project for scientific and technological innovation in the health industry of Gansu Province (GSWSZD-2024-17), General Project of Gansu Province Joint Research Fund (24JRRA929), Key Talents Project of Gansu Province (2019RCXM020), Science and Technology Project of Chengguan District of Lanzhou City (2020SHFZ0039, 2020JSCX0073), Medical Research Innovation Ability Improvement Project of Lanzhou University (lzuyxcx-2022-160), Excellent Textbook Cultivation Project of Lanzhou University (lzuyxcx-2022-45).
Author information
Authors and Affiliations
Contributions
All authors listed have contributed to this work.
Corresponding author
Ethics declarations
Consent for Publication
They have given their informed consent for this article to be published.
Ethics Approval and Consent to Participate
Not applicable
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, L., Liu, B., Wang, Z. et al. Evolving Landscape of HER2-Targeted Therapies for Gastric Cancer Patients. Curr. Treat. Options in Oncol. 26, 260–277 (2025). https://doi.org/10.1007/s11864-025-01300-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-025-01300-0



