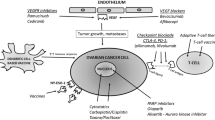
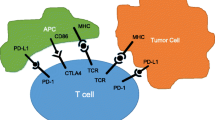
Opinion statement
The rise of immunotherapy is the greatest advance in oncology to occur over the last several years, but applications in gynecologic malignancies lag behind other tumors. The term “immunotherapy” envelops monoclonal antibodies as receptor mediators, including immune checkpoint inhibitors (ICPI), cancer vaccines, and adoptive immunotherapies alone or in combination with other therapeutic approaches. The purpose of this review is to summarize the status of immunotherapy trials in ovarian cancer and to specifically highlight data published in the last 1–2 years.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ovarian cancer—cancer stat facts [Internet]. [cited 2018 May 21]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, Vargas-Aguilar VM. Management of recurrent epithelial ovarian cancer. Gland Surg. 2014;3(3):198–202.
Marth C, Reimer D, Zeimet AG. Front-line therapy of advanced epithelial ovarian cancer: standard treatment. Ann Oncol. 2017;28(suppl_8):viii36–9.
Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53.
National Comprehensive Cancer Network. Ovarian Cancer v 2.2018.
National Institutes of Health. Surveillance, epidemiology, end results: ovarian cancer.
Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia | NEJM [Internet]. 2011 [cited 2018 May 22]. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1103849
Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018 [cited 2018 May 22]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa1716078?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov
Trastuzumab after adjuvant chemotherapy in HER2-Positive breast cancer | NEJM 2005 [Internet]. [cited 2018 May 22]. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa052306
Clarke B, Tinker AV, Lee C-H, Subramanian S, van de Rijn M, Turbin D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22(3):393–402.
Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer | NEJM [Internet]. 2003 [cited 2018 May 22]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa020177?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–43.
Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8.
Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25–39.
Mocellin S, Benna C, Pilati P. Coinhibitory molecules in cancer biology and therapy. Cytokine Growth Factor Rev. 2013;24(2):147–61.
Fife BT, Bluestone JA. Control of peripheral T cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82.
Krummel MF, Allison JP. Pillars article: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of Experimental Medicine. 1995. 182: 459–465. J Immunol. 2011;187(7):3459–65.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Fessas P, Lee H, Ikemizu S, Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44(2):136–40.
•• Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity. JCO. 2016;34(15_suppl):5533 Available from: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.5533. A preliminary analysis for trial of avelumab in patients with recurrent or refractory ovarian cancer. Responses for patients that were PD-L1+ were compared with PD-L1- expression.
Phase II study of ipilimumab monotherapy in recurrent platinum-sensitive ovarian cancer—study results - ClinicalTrials.gov [Internet]. [cited 2018 Mar 28]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01611558. A trial to investigate the overall response and adverse events related to ipilimumab as a monotherapy for recurrent ovarian cancer.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;14:JCO2017776385.
Safety and activity of anti-PD-L1 antibody in patients with advanced cancer | NEJM [Internet]. 2012 [cited 2018 May 25]. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1200694
Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Mar 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT02657889
PEMBRO with chemo in neo adj treatment of ovarian cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT03275506
A phase II study of nivolumab/bevacizumab - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT02873962
A study in ovarian cancer patients evaluating rucaparib and nivolumab as maintenance treatment following response to front-line platinum-based chemotherapy - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT03522246
ATALANTE: atezolizumab vs placebo phase III study in late relapse ovarian cancer treated with chemotherapy + bevacizumab - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT02891824
Lee J-M, Cimino-Mathews A, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. JCO. 2017;35(19):2193–202.
A Study of atezolizumab versus placebo in combination with paclitaxel, carboplatin, and bevacizumab in participants with newly-diagnosed stage iii or stage iv ovarian, fallopian tube, or primary peritoneal cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Mar 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT03038100
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology [Internet]. 2017 Jan 6 [cited 2018 Apr 2];6(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5353914/
Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–98.
Bellone S, Buza N, Choi J, Zammataro L, Gay L, Elvin J, et al. Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clinical Cancer Research [Internet]. 2018 Jan 19 [cited 2018 Jun 6]; Available from: http://clincancerres.aacrjournals.org/lookup/doi/10.1158/1078-0432.CCR-17-1805
Olaparib, durvalumab, and tremelimumab in treating patients with recurrent or refractory ovarian, fallopian tube or primary peritoneal cancer with BRCA1 or BRCA2 mutation - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT02953457
Pembrolizumab in treating participants with metastatic, recurrent or locally advanced cancer and genomic instability - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT03428802
Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang X-Y. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–75.
Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M, et al. Phase I trial of “bi-shRNAifurin/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20(3):679–86 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3293620/.
Manning L, Barve M, Wallraven G, Kumar P, Taquet N, Bognar E, et al. Assessment of low dose Vigil® engineered autologous tumor cell (EATC) immunotherapy in patients with advanced solid tumors. Clin Oncol. 2017;2:4.
Trial of Adjuvant FANG™ vaccine for high risk stage III/IV ovarian cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT01309230
Phase 2 trial of maintenance vigil for high risk stage IIIb-IV ovarian cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT02346747
Vreeland TJ, Litton JK, Qiao N, Philips AV, Alatrash G, Hale DF, et al. Phase Ib trial of folate binding protein (FBP)-derived peptide vaccines, E39 and an attenuated version, E39’: an analysis of safety and immune response. Clin Immunol. 2018; Available from: http://www.sciencedirect.com/science/article/pii/S1521661617308653.
Kalli KR, Block MS, Kasi PM, Erskine CL, Hobday TJ, Dietz A, et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. Clin Cancer Res. 2018. A phase I study that demonstrates that generation of an immune response to folate receptor vaccination occurs in a large number of patients in clinical remission and remains detectable at 1 year.
PH3 study of mirvetuximab soravtansine vs investigator’s choice of chemotherapy in women with Fra+ Adv. EOC, primary peritoneal or fallopian tube cancer - full text view - ClinicalTrials.gov [Internet]. [cited 2018 Jun 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT02631876
Altwerger G, Bonazzoli E, Bellone S. In Vitro and in vivo activity of IMGN853, an antibody–drug conjugate targeting folate receptor alpha linked to DM4, in biologically aggressive endometrial cancers. Mol Cancer Ther. 2018; Available from: http://mct.aacrjournals.org/content/17/5/1003.long.
Ab O, Whiteman KR, Bartle L. IMGN853, a folate receptor-α (FRα)–targeting antibody–drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol Cancer Ther. 2015; Available from: http://mct.aacrjournals.org/content/14/7/1605.long.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15.
Odunsi K. Immunotherapy in ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii1–7 Available from: https://academic.oup.com/annonc/article/28/suppl_8/viii1/4693810.
Battaglia A, Fossati M, Buzzonetti A, Scambia G, Fattorossi A. A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients. Immunol Lett. 2017;191:35–9.
• Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, et al. p53-reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin Cancer Res. 2018;24(6):1315–25 Available from: http://clincancerres.aacrjournals.org.proxy-hs.researchport.umd.edu/content/24/6/1315. A phase I trial to demonstrate that response to p53 vaccination in conjunction with cytotoxic chemotherapy correlates with longer PFS.
Mittica G, Capellero S, Genta S, Cagnazzo C, Aglietta M, Sangiolo D, et al. Adoptive immunotherapy against ovarian cancer. J Ovarian Res. 2016;9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869278/.
Krishnan V, Berek JS, Dorigo O. Immunotherapy in ovarian cancer. Curr Probl Cancer. 2017;41(1):48–63.
Uppendahl LD, Dahl CM, Miller JS, Felices M, Geller MA. Natural killer cell-based immunotherapy in gynecologic malignancy: a review. Front Immunol. 2018;8 Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01825/full.
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–9.
Yang Y, Lim O, Kim TM, Ahn Y-O, Choi H, Chung H, et al. Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4(3):215–24.
Eguizabal C, Zenarruzabeitia O, Monge J, Santos S, Vesga MA, Maruri N, et al. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front Immunol. 2014;5:439.
Klapdor R, Wang S, Hacker U, Büning H, Morgan M, Dörk T, et al. Improved Killing of ovarian cancer stem cells by combining a novel chimeric antigen receptor-based immunotherapy and chemotherapy. Hum Gene Ther. 2017;28(10):886–96.
Martín-Antonio B, Suñe G, Perez-Amill L, Castella M, Urbano-Ispizua A. Natural killer cells: angels and devils for immunotherapy. Int J Mol Sci. 2017;29:18(9).
Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, et al. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother. 2014;37(2):115–22.
Zhang Z, Wang L, Luo Z, Zhao X, Huang J, Li H, et al. Efficacy and safety of cord blood-derived cytokine-induced killer cells in treatment of patients with malignancies. Cytotherapy. 2015;17(8):1130–8.
Zhang C, Zhang Z, Wang L, Han J, Li F, Shen C, et al. Pseudomonas aeruginosa-mannose sensitive hemagglutinin injection treated cytokine-induced killer cells combined with chemotherapy in the treatment of malignancies. Int Immunopharmacol. 2017;51:57–65.
Rodriguez-Garcia A, Minutolo NG, Robinson JM, Powell DJ. T cell target antigens across major gynecologic cancers. Gynecol Oncol. 2017;145(3):426–35.
Andersen R, Donia M, Westergaard MCW, Pedersen M, Hansen M, Svane IM. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma. Hum Vaccin Immunother. 2015;11(12):2790–5.
Deniger DC, Pasetto A, Robbins PF, Gartner JJ, Prickett TD, Paria BC, et al. T-cell responses to TP53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res. 2018. In this paper, TILs from ovarian cancer patients were found to have specificity to mutated neoantigens and that these T cells could be used for adoptive cell therapy. TP53 “hotspot” reactive T cells were also found and these cells could recognize a broad range of tumor types in unrelated individuals.
Bobisse S, Genolet R, Roberti A, Tanyi JL, Racle J, Stevenson BJ, et al. Sensitive and frequent identification of high avidity neo-epitope specific CD8+ T cells in immunotherapy-naive ovarian cancer. Nat Commun. 2018. 1092;9(1):15.
Pedersen M, Westergaard M, Nielsen M, Borch TH, Poulsen LG, Hendel H, et al. 1145PDAdoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic ovarian cancer: a pilot study. Ann Oncol. 2017;28(suppl_5) Available from: https://academic.oup.com/annonc/article/28/suppl_5/mdx376.010/4109224.
Fishman MN, Thompson JA, Pennock GK, Gonzalez R, Diez LM, Daud AI, et al. Phase I trial of ALT-801, an interleukin-2/T cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011;17(24):7765–75.
Zhu X, Cai H, Zhao L, Ning L, Lang J, Zhu X, et al. CAR-T cell therapy in ovarian cancer: from the bench to the bedside. Oncotarget. 2017;8(38):64607–21.
Tanyi JL, Haas AR, Beatty GL, Stashwick CJ, O’Hara MH, Morgan MA, et al. Anti-mesothelin chimeric antigen receptor T cells in patients with epithelial ovarian cancer. JCO. 2016;34(15_suppl):5511.
Lanitis E, Dangaj D, Hagemann IS, Song D-G, Best A, Sandaltzopoulos R, et al. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS ONE. 2012;7(11):e49829.
Tanyi JL, Stashwick C, Plesa G, Morgan MA, Porter D, Maus MV, et al. Possible compartmental cytokine release syndrome in a patient with recurrent ovarian cancer after treatment with mesothelin-targeted CAR-T cells. J Immunother. 2017;40(3):104–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Fan, C.(., Reader, J. & Roque, D.M. Review of Immune Therapies Targeting Ovarian Cancer. Curr. Treat. Options in Oncol. 19, 74 (2018). https://doi.org/10.1007/s11864-018-0584-3
Published:
DOI: https://doi.org/10.1007/s11864-018-0584-3
Keywords
- Ovarian cancer
- Immunotherapy
- Immune checkpoint inhibitors
- Cancer vaccine
- Adoptive immunotherapy
- PD1
- PD-L1
- PD-L2
- CTLA-4
- Nivolumab
- Ipilimumab
- Pembrolizumab
- Avelumab
- Atezolizumab
- PARP inhibitors
- BRCA 1/2 mutation
- Microsatellite instability
- Bevacizumab
- Anti-angiogenic
- NY-ESO
- MAGE
- p53
- Mirvetuximab soravtansine
- Folate receptor alpha
- Folate-binding protein
- TILS
- CAR-T cells
- NK cells
- T cells
- HER2
- Mesothelin