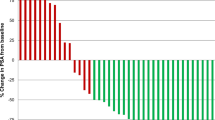
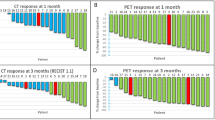
Opinion statement
Patients with unresectable hepatic colorectal metastases who become chemo-refractory have limited treatment options. Systemic chemotherapies such as TAS102 and regorafenib have been used in the refractory setting, but with only modest improvement in overall survival compared to best supportive care. In patients with liver-only or liver-dominant disease, direct chemotherapy to the liver such as hepatic artery infusional (HAI) chemotherapy and radioembolization (yttrium-90 (Y90)) should be considered. Due to the difficulty of HAI therapy post Y90 for technical reasons, we recommend HAI therapy prior to Y90.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
•• D'Angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261:353–60. This study demonstrated the benefit of the addition of HAI therapy to systemic chemotherapy in conversion of unresectable liver metastases to resectable
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64.
• Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19. TAS 102 demonstrated a survival benefit of 1.5 months versus best supportive care in patients with refractory metastatic colorectal cancer in this phase III randomized trial
• Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. In a large phase III study of regorafenib versus best supportive care in chemotherapy refractory metastatic colorectal cancer, the use of regorafenib led to a 1.5-month survival benefit
Kemeny N, Conti JA, Cohen A, Campana P, Huang Y, Shi WJ, et al. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 1994;12:2288–95.
Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–71.
Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol. 2015;22:794–802.
Nosher JL, Ohman-Strickland PA, Jabbour S, Narra V, Nosher B. Changes in liver and spleen volumes and liver function after radioembolization with yttrium-90 resin microspheres. J Vasc Interv Radiol. 2011;22:1706–13.
Salem R, Thurston KG, Carr BI, Goin JE, Geschwind JF. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13:S223–9.
Cercek Andrea, Kemeny NE. Chapter 11 hepatic arterial infusion pumps In: Goodman MD ed Regional therapeutics for advanced malignancies: Jaypee Brothers Pvt Ltd, 2012.
Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395–403.
Kemeny N, Seiter K, Niedzwiecki D, Chapman D, Sigurdson E, Cohen A, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327–33.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80.
Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–24.
Heinemann V, Volker H, von Ludwig Fischer W, Thomas D, Alexander K. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Venook AP, Niedzwiecki D, Lenz HG, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). Proc Am Soc Clin Oncol 2014; 32 (suppl): LBA3 (abstr).
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Heinemann V, Modest DP, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges TB, Lerchenmuller CA, Kahl C, SEipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Geissen CA, Jung A, Kirchner T, Stintzing S. Gender and tumor location as predictors for efficacy: influence on endpoints in the first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol. 2014;23:5s. suppl; abstr 3600
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2016; doi:10.1001/jamaoncol.2016.3797.
Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–48.
Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–5.
Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459–65.
Sigurdson ER, Ridge JA, Kemeny N, Daly JM. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol. 1987;5:1836–40.
Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2:498–504.
•• Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, Kingham TP, Allen PJ, Fong Y, Cercek A, D’Angelica MI. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113(5):477–84. In patients with resected liver metastases, adjuvant treatment with HAI plus systemic chemotherapy has produced 5-year survival as high as 78%
Kemeny N, Gonen M, Sullivan D, Schwartz L, Benedetti F, Saltz L, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19:2687–95.
•• van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland AH, Ferguson T, Rodríguez J, Kröning H, Wolf I, Ganju V, Walpole E, Boucher E, Tichler T, Shacham-Shmueli E, Powell A, Eliadis P, Isaacs R, Price D, Moeslein F, Taieb J, Bower G, Gebski V, Van Buskirk M, Cade DN, Thurston K, Gibbs P. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(15):1723–31. doi:10.1200/JCO.2015.66.1181. Patients treated with first-line y90 SIRT in combination with FOLFOX had a significantly longer hepatic disease-free interval compared to those who received chemotherapy alone, but there was no difference in overall survival.
Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE Jr, Loehr SP, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–25.
Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–31.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(309–318):1999. discussion 318–321
Sofocleous CT, Sideras PA, Petre EN. Interventional management of colorectal cancer liver metastases. In: Kee ST, Madoff DC, Murthy R, editors. Clinical interventional oncology. 1st ed. Philadelphia: Elsevier Saunders; 2014. p. 135–43.
Kemeny N, Kemeny M, Dawson L. Liver metastases. In: Abeloff MD, Armitage JO, Niderhuber JE, Kastan MB, Gilles McKenna W, editors. Clinical oncology, vol. Chap. 59. New York: Churchill Livingstone, Inc.; 2008. p. 885–923.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Andrea Cercek declares that she has no conflict of interest.
Vyacheslav Gendel declares that he has no conflict of interest.
Salma Jabbour declares that she has no conflict of interest.
Dirk Moore declares that he has no conflict of interest.
Chunxia Chen declares that she has no conflict of interest.
John Nosher declares that he has no conflict of interest.
Marinela Capanu declares that she has no conflict of interest.
Joanne F. Chou declares that she has no conflict of interest.
Taryn Boucher declares that she has no conflict of interest.
Nancy Kemeny has received financial support through a grant from Amgen.
Darren R. Carpizo has received financial support through a grant and has received compensation from Z53 Therapeutics for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Research Funding
MSK Cancer Center Support Grant/Core Grant (P30 CA008748), R01CA200800, K08CA172676, BCRF to DRC.
Additional information
This article is part of the Topical Collection on Lower Gastrointestinal Cancers
This paper is not based on previous communication to a society or meeting.
Rights and permissions
About this article
Cite this article
Cercek, A., Gendel, V., Jabbour, S. et al. A Comparison of Yttrium-90 Microsphere Radioembolization to Hepatic Arterial Infusional Chemotherapy for Patients with Chemo-refractory Hepatic Colorectal Metastases. Curr. Treat. Options in Oncol. 18, 42 (2017). https://doi.org/10.1007/s11864-017-0481-1
Published:
DOI: https://doi.org/10.1007/s11864-017-0481-1