Abstract
Objective
Histone deacetylase 4 (HDAC4) regulates lipid accumulation, inflammation, endothelial injury, and atherosclerosis to participate in the pathogenesis of cardiovascular diseases. This study aimed to explore the value of serum HDAC4 change before and after percutaneous coronary intervention (PCI) in predicting major adverse cardiovascular events (MACE) risk in acute coronary syndrome (ACS) patients.
Methods
HDAC4 from serum was detected by enzyme-linked immunosorbent assay in 340 ACS patients at baseline, day (D)1, D3, and D7 after PCI, and from 30 healthy controls (HCs). MACE was recorded during follow-up.
Results
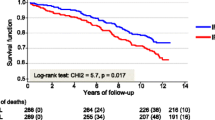
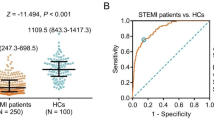
HDAC4 was decreased in ACS patients versus HCs (P < 0.001). In ACS patients, HDAC4 was negatively related to total cholesterol (P = 0.025), low-density lipoprotein cholesterol (P = 0.007), C-reactive protein (P < 0.001), cardiac troponin I (P < 0.001), and hyperlipidemia history (P = 0.015). Additionally, HDAC4 was lowest in ST-elevation myocardial infarction (STEMI) patients, followed by non-STEMI patients, and highest in unstable angina patients (P = 0.010). After PCI, HDAC4 was decreased from baseline to D1, then increased until D7 (P < 0.001). Furthermore, HDAC4 at baseline (P = 0.002), D1 (P < 0.001), D3 (P < 0.001), and D7 (P < 0.001) were all reduced in patients who experienced MACE versus those who did not. Meanwhile, high HDAC4 at baseline (P = 0.036), D1 (P = 0.010), D3 (P = 0.012), and D7 (P = 0.012) estimated decreased accumulating MACE risk by Kaplan–Meier curve. Multivariate logistic analysis revealed that HDAC4 at D1 was independently linked to lower MACE risk (odds ratio = 0.957, P = 0.039).
Conclusion
Serum HDAC4 is decreased from baseline to D1, then elevated until D7, and its increased level correlates with lower MACE risk in ACS patients receiving PCI.




Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bergmark BA, Mathenge N, Merlini PA et al (2022) Acute coronary syndromes. Lancet 399(10332):1347–1358
Bhatt DL, Lopes RD, Harrington RA (2022) Diagnosis and treatment of acute coronary syndromes: A review. JAMA 327(7):662–675
McConaghy JR, Sharma M, Patel H (2020) Acute chest pain in adults: outpatient evaluation. Am Fam Physician 102(12):721–727
Kamran H, Jneid H, Kayani WT et al (2021) Oral antiplatelet therapy after acute coronary syndrome: A review. JAMA 325(15):1545–1555
Smith JN, Negrelli JM, Manek MB et al (2015) Diagnosis and management of acute coronary syndrome: an evidence-based update. J Am Board Fam Med 28(2):283–293
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Vogel B, Claessen BE, Arnold SV et al (2019) ST-segment elevation myocardial infarction. Nat Rev Dis Primers 5(1):39
Collet JP, Thiele H, Barbato E et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42(14):1289–1367
Gaudino M, Hameed I, Farkouh ME et al (2020) Overall and cause-specific mortality in randomized clinical trials comparing percutaneous interventions with coronary bypass surgery: A meta-analysis. JAMA Intern Med 180(12):1638–1646
Zhang S, Wang W, Sawhney JPS et al (2020) Antithrombotic management and long-term outcomes following percutaneous coronary intervention for acute coronary syndrome in Asia. Int J Cardiol 310:16–22
Choi MC, Ryu S, Hao R et al (2014) HDAC4 promotes Pax7-dependent satellite cell activation and muscle regeneration. EMBO Rep 15(11):1175–1183
Usui T, Okada M, Mizuno W et al (2012) HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am J Physiol Heart Circ Physiol 302(9):H1894-1904
Yang D, Xiao C, Long F et al (2018) HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc Res 114(7):1016–1028
Yang Y, Wang Z, Xu Y et al (2022) Knockdown of lncRNA H19 alleviates ox-LDL-induced HCAECs inflammation and injury by mediating miR-20a-5p/HDAC4 axis. Inflamm Res 71(9):1109–1121
Zhang F, Cheng N, Du J et al (2021) MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc Disord 21(1):172
Chen M, Cheng H, Chen X et al (2023) The activation of histone deacetylases 4 prevented endothelial dysfunction: A crucial mechanism of HuangqiGuizhiWuwu Decoction in improving microcirculation dysfunction in diabetes. J Ethnopharmacol 116240
Chen F, Li J, Zheng T et al (2022) KLF7 Alleviates atherosclerotic lesions and inhibits glucose metabolic reprogramming in macrophages by regulating HDAC4/miR-148b-3p/NCOR1. Gerontology 68(11):1291–1310
Greenwood JP, Ripley DP, Berry C et al (2016) Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE Guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA 316(10):1051–1060
Wang M, Pang X, Lu H, Wang X (2022) Clinical role of serum histone deacetylase 4 measurement in acute ischemic stroke: Relation to disease risk, severity, and prognosis. J Clin Lab Anal 36(5):e24372
Mou X, Jin Y, Jin D et al (2022) Serum HDAC4 level in rheumatoid arthritis: Longitudinal change during treatment and correlation with clinical outcomes. J Clin Lab Anal 36(8):e24594
Dou B, Ma F, Jiang Z, Zhao L (2022) Blood HDAC4 variation links with disease activity and response to tumor necrosis factor inhibitor and regulates CD4+ T cell differentiation in ankylosing spondylitis. Front Med (Lausanne) 9:875341
Wang B, Moya N, Niessen S et al (2011) A hormone-dependent module regulating energy balance. Cell 145(4):596–606
Liu ZM, Wang X, Li CX et al (2022) SP1 promotes HDAC4 expression and inhibits HMGB1 expression to reduce intestinal barrier dysfunction, oxidative stress, and inflammatory response after sepsis. J Innate Immun 14(4):366–379
Li L, Fu W, Gong X et al (2021) The role of G protein-coupled receptor kinase 4 in cardiomyocyte injury after myocardial infarction. Eur Heart J 42(14):1415–1430
Tucker B, Vaidya K, Cochran BJ, Patel S (2021) Inflammation during percutaneous coronary intervention-prognostic value, mechanisms and therapeutic targets. Cells 10(6)
Liu J, Zhou X, Li Q et al (2017) Role of phosphorylated HDAC4 in stroke-induced angiogenesis. Biomed Res Int 2017:2957538
Hao J, Chen Y, Yu Y (2022) Circular RNA circ_0008360 inhibits the proliferation, migration, and inflammation and promotes apoptosis of fibroblast-like synoviocytes by regulating miR-135b-5p/HDAC4 axis in rheumatoid arthritis. Inflammation 45(1):196–211
Shanaki M, Omidifar A, Shabani P, Toolabi K (2022) Association between HDACs and pro-inflammatory cytokine gene expressions in obesity. Arch Physiol Biochem 128(4):880–886
Funding
This study was supported by the Medical Science and Technology Joint Construction Project of Henan Province (LHGJ20221018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approval from the Ethics Committee was obtained, and written informed consent from each subject was also gained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Zhang, J., Jia, H. et al. Serum histone deacetylase 4 longitudinal change for estimating major adverse cardiovascular events in acute coronary syndrome patients receiving percutaneous coronary intervention. Ir J Med Sci 192, 2689–2696 (2023). https://doi.org/10.1007/s11845-023-03326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03326-5