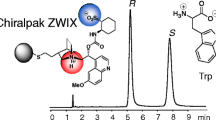
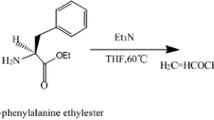
Abstract
The discrimination of enantiomers is a challenging task in separation technology, and using a membrane is most promising for separating enantiomers from racemic mixture. The optical resolution of chiral compounds is of interest to researchers working in a variety of fields from analytical, organic and medicinal chemistry, to pharmaceutics and materials, to process engineering for fabricating pharmaceuticals, agrochemicals, fragrances and foods, and so on. There is considerable demand for separation techniques appropriate for the large-scale resolution of chiral molecules. The separation of chiral compounds using chiral or achiral/non-chiral polymeric membranes with or without chiral selector represents a promising system for future commercial applications. This review focuses on an active field of chiral separation, membrane-based enantioseparation technique, which has potential for large-scale production of singleenantiomers. Enantiomeric separation by membrane processes has been studied using various configurations of liquid and solid polymer membranes. Selectivity and permeability of liquid-membranes is reasonably good because the rate of diffusion of solute molecules is high in liquids but has inferior durability and stability. Solid polymer membranes have inferior permeability because diffusion of solute through solids is slow but quite stable and durable; however, commercial application of membrane technology for optical resolution is yet to be realized. Several chiral separation membranes were prepared from chiral polymers where enantioselectivity was generated from chiral carbons in the main chain. However, it is rather tricky to generate excellent chiral separation membranes from chiral polymers alone, because racemic penetrants mainly encounter the flexible side chains of the membrane polymers.
Similar content being viewed by others
References
P.G. Ingole, N. R. Thakare, K. H. Kim, H.C. Bajaj, K. Singh and H. K. Lee, New J. Chem., 37, 4018 (2013).
N. M. Maier, P. Franco and W. Linder, J. Chromatogr. A, 906, 3 (2001).
J.M. Daniels, E. R. Nestmann and A. Kerr, J. Drug Inf., 31, 639 (1997).
A. Ghanem and H.Y. Aboul-Enein, Tetrahedron: Asym., 15, 3331 (2004).
A. Ghanem and H. Y. Aboul-Enein, Chirality, 17, 1 (2005).
J.M. Keith, J. F. Larrow and E.N. Jacobsen, Adv. Synth. Catal., 343, 5 (2001).
H. E. Schoemaker, D. Mink and M.G. Wubbolts, Science, 299, 1694 (2003).
U. T. Bornscheuer, Angew. Chem. Int. Ed., 42, 3336 (2003).
H. Pellissier, Tetrahedron, 59, 8291 (2003).
U. T. Strauss, U. Felfer and K. Faber, Tetrahedron: Asym., 10, 107 (1999).
U. T. Strauss and K. Faber, Tetrahedron: Asym., 10, 4079 (1999).
G. L. J. A. Rikken and E. Raupach, Nature, 405, 932 (2000).
G. M. Maggiora, B. Mao and K. C. Chou, New Developments in Molecular Chirality, Ed. P. G. Mezey, Kluwer Academic Publishers, Dordrecht., 5, 93 (1991).
W. O. Foye, T. L. Lemke and D. A. Williams, Foye’s principles of medicinal chemistry, Lippincott Williams & Wilkins (2007).
I. Aranaz, M. Mengíbar, R. Harris, I. Paños, B. Miralles, N. Acosta, G. Galed and Á. Heras, Current Chem. Biolo., 3, 203 (2009).
I. I. Hewala, M. S. Moneeb, H. A. Elmongy and A.-A. M. Wahbi, Talanta, 130, 506 (2014).
H. Caner, E. Groner and L. Levy, Drug Discovery Today, 9, 105 (2004).
Anon, FDA’s Policy statement for the development of new stereoisomeric drugs, Chirality, 4, 338 (1992).
J. Tobis, L. Bocha, Y. Thomanna and J.C. Tiller, J. Membr. Sci., 372, 219 (2011).
Food & Drug Administration, Policy Statement for the Development of New Stereoisomeric Drugs, 57 Fed. Reg., 22, 249 (1992).
N. M. Maier, P. Franco and W. Lindner, J. Chromatogr. A, 906, 3 (2001).
C. A.M. Afonso and J.G. Crespo, Angew. Chem., Int. Ed., 43, 5293 (2004).
K. B. Jirage and C. R. Martin, Trends Biotechnol., 17, 197 (1999).
P.M. Masters, Forensic Sci. Int., 32, 179 (1986).
J. R. Cronin and S. Pizzarello, Science, 275, 951 (1997).
M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Kesseler, R. Sturmer and T. Zelinski, Angew. Chem., Int. Ed., 43, 788 (2004).
A. R. Fassihi, Int. J. Pharm., 92, 1 (1993).
R. Sabia, A. Ciogli, M. Pierini, F. Gasparrini and C. Villani, J. Chromatogr. A (2014), http://dx.doi.org/10.1016/j.chroma.2014.07.097.
W. R. Brode, J. Opt. Soc. Am., 41, 987 (1951).
T. J. Ward and T.M. Oswald, Encyclopaedia of Analytical Chemistry (2006).
C.M. Kraml, D. Zhou, N. Byrne and O. McConnell, J. Chromatogr. A, 1100, 108 (2005).
H. Nohira, K. Watanabe and M. Kurokawa, Chem. Lett., 5, 299 (1976).
J. Jacques and A. Collet, Wiley Interscience, New York (1981).
B. S. Sekhon, Int. J. Chem. Technol. Res., 2, 1584 (2010).
A. S. Bommarius and B. R. Riebel-Bommarius, Biocatalysis: Fundamentals and Applications, Wiley (2007).
E. Lee, M.B. Park, J.M. Kim, W. S. Kim and I. H. Kim, Korean J. Chem. Eng., 27, 231 (2010).
R. D. Noble, J. Membr. Sci., 75, 121 (1992).
M. Ulbricht, Polymer, 7, 2217 (2006).
M. Nakamura, S. Kiyohara, K. Saito, K. Sugita and T. Sugo, Anal. Chem., 71, 1323 (1999).
N. H. Lee and C.W. Frank, Polymer, 43, 6255 (2002).
M. Newcomb, R. C. Helgeson and D. J. Cram, J. Am. Chem. Soc., 96, 7367 (1974).
A. Maruyama, N. Adachi, T. Takatsuki, M. Torii, K. Sanui and N. Ogata, Macromolecules, 23, 2748 (1990).
T. Aoki, K. Shinohara, T. Kaneko and E. Oikawa, Macromolecules, 29, 4192 (1996).
T. Aoki, Y. Kobayashi, T. Kaneko, E. Oikawa, Y. Yamamura, Y. Fujita, M. Teraguchi, R. Nomura and T. Masuda, Macromolecules, 32, 79 (1999).
T. Aoki, Prog. Polym. Sci., 24, 951 (1999).
T. Aoki, M. Ohshima, K. Shinohara, T. Kaneko and E. Oikawa, Polymer, 38, 235 (1997).
M. Teraguchi and T. Masuda, Macromolecules, 35, 1149 (2002).
M. Teraguchi, J. Suzuki, T. Kaneko, T. Aoki and T. Masuda, Macromolecules, 36, 9694 (2003).
M. Teraguchi, K. Mottate, S.Y. Kim, T. Aoki, T. Kaneko, S. Hadano and T. Masuda, Macromolecules, 38, 6367 (2005).
Y. Masakazu and H. Akon, Enantioselective Membranes, DOI: 10.1002/9781118522318.emst131 John Wiley & Sons, Inc. All (2013).
H. K. Lonsdale, J. Membr. Sci., 10, 81 (1982).
S.V. Madihally, Principles of Biomedical Engineering, Artech House, Boston London (2010).
J. P.G. Villaluenga and A. Tabe-Mohammadi, J. Membr. Sci., 169, 159 (2000).
J. E. Cadotte, K. E. Cobian, R. H. Forester and R. J. Petersen, NTIS Report No. PB-253193 (1976).
S. A. Altinkaya, Desalination, 199, 459 (2006).
K. Singh, P.G. Ingole, H.C. Bajaj, A. Bhattacharya and H.R. Brahmbhatt, Sep. Sci. Technol., 45, 1374 (2010).
E. Vedejs and M. Jure, Angew. Chem., Int. Ed., 44, 3974 (2005).
G. R. Cook, Curr. Org. Chem., 4, 869 (2000).
J.M. Keith, J. F. Larrow and E.N. Jacobsen, Adv. Synth. Catal., 343, 5 (2001).
M. Tokunaga, J. F. Larrow, F. Kakiuchi and E. N. Jacobsen, Science, 277, 936 (1997).
S. Ramdeehul, P. Dierkes, R. Aguado, P. C. J. Kamer, P.W.N.M. Van Leeuwen and J. A. Osborn, Angew. Chem., Int. Ed., 37, 3118 (1998).
H. Pellissier, Tetrahedron, 59, 8291 (2003).
V. Schurig, J. Chromatogr. A, 906, 275 (2001).
S. Fanali, P. Catarcini, G. Blaschke and B. Chankvetadze, Electrophoresis, 22, 3131 (2001).
Y. Nagata, T. Iida and M. Sakai, J. Mol. Catal. B: Enzym., 12, 105 (2001).
S. S. Peacock, D.M. Walba, F.C. A. Gaeta, R.C. Helgeson and D. J. Cram, J. Am. Chem. Soc., 102, 2043 (1980).
B. Dietrich, J. M. Lehn and J. P. Sauvage, Tetrahedron, 29, 1647 (1973).
B. Dietrich and J.M. Lehn, Tetrahedron Lett., l5, 1225 (1973).
E. P. Kyba, J.M. Timko, L. J. Kaplan, F. de Jong, G.W. Gokel and D. J. Cram, J. Am. Chem. Soc., 100, 4555 (1978).
D. J. Cram and J. H. Cram, ACC. Chem. Res., 11, 8 (1978).
L. R. Sousa, D. H. Hoffman, L. Kaplan and D. J. Cram, J. Am. Chem. Soc., 96, 7100 (1974).
L.R. Sousa, G. D.Y. Sogah, D. H. Hoffmann and D. J. Cram, J. Am. Chem. Soc., 100, 4569 (1978).
G.D.Y. Sogah and D. J. Cram, J. Am. Chem. Soc., 101, 3035 (1979).
G. D.Y. Sough and D. J. Cram, J. Am. Chem. Soc., 97, 1259 (1975).
W. H. Pirkle, US Patent, 5,080,795 (1992).
W. H. Pirkle, WO 9,117,816 (1992).
W. H. Pirkle and W. E. Bowen, Tetrahedron: Asymmetry, 5, 773 (1994).
W. H. Pirkle and E.M. Doherty, J. Am. Chem. Soc., 111, 4113 (1989).
C. Thoelen, M. De Bruyn, E. Theunissen, Y. Kondo, I. F. J. Vankelecom, P. Grobet, M. Yoshikawa and P. A. Jacobs, J. Membr. Sci., 186, 153 (2001).
T. Aoki, S. Tomizawa and E. Oikawa, J. Membr. Sci., 99, 117 (1995).
E. Yashima, J. Noguchi and Y. Okamoto, J. Appl. Polym. Sci., 54, 1087 (1994).
T. Kakuchi, T. Takaoka and K. Yokota, Polym. J., 22, 199 (1990).
Y. Xiao, H.M. Lim, T. S. Chung and R. Rajagopalan, Langmuir, 23, 12990 (2007).
Z. Zhou, Y. Xiao, T. A. Hatton and T. S. Chung, J. Membr. Sci., 339, 21 (2009).
Y. Zhang, H. Yu, Y. Wu, W. Zhao, M. Yang, H. Jing and A. Chen, Ana. Biochem., 462, 13 (2014).
K. He, F. Qiu, J. Qin, J. Yan and D. Yang, Korean J. Chem. Eng., 30, 2078 (2013).
X. Liu and L. Meng, Korean J. Chem. Eng., 30, 918 (2013).
J. H. Kim, J. H. Kim, J. Jegal and K.H. Lee, J. Membr. Sci., 213, 273 (2003).
W. Li, Y. Li, Y. Fu and J. Zhang, Korean J. Chem. Eng., 30, 1448 (2013).
L. Chen, X. He, B. Zhao and Y. Liu, Anal. Chim. Acta, 417, 51 (2000).
N. Sunsandee, N. Leepipatpiboon, P. Ramakul, T. Wongsawa and U. Pancharoen, Sep. Purif. Technol., 102, 50 (2013).
N. Sunsandee, N. Leepipatpiboon and P. Ramakul, Korean J. Chem. Eng., 30, 1312 (2013).
A. Higuchi, M. Hara, T. Horiuchi and T. Nakagawa, J. Membr. Sci., 93, 157 (1994).
K. Shinohara, T. Aoki and E. Oikawa, Polymer, 36, 2403 (1995).
K. Sakaki, S. Hara and N. Itoh, Desalination, 149, 247 (2002).
Y. J. Wang, Y. Hu, J. Xu, G. S. Luo and Y.Y. Dai, J. Membr. Sci., 293, 133 (2007).
A. Bodalo, J.L. Gomez, E. Gomez, M. F. Maximo and M. C. Montiel, Enzyme Microb. Technol., 35, 261 (2004).
A. Liese, U. Kragl, H. Kierkels and B. Schulze, Enzyme Microb. Technol., 30, 673 (2002).
W. S. Long, S. Bhatia and A. Kamaruddin, J. Membr. Sci., 219, 69 (2003).
E. Miyako, T. Maruyama, N. Kamiya and M. Goto, Chem. Eur. J., 11, 1163 (2005).
L.M. Robeson, B. D. Freeman, D. R. Paul and B.W. Rowe, J. Membr. Sci., 341, 178 (2009).
L.M. Robeson, J. Membr. Sci., 320, 390 (2008).
B. D. Freeman, Macromolecules, 32, 375 (1999).
W. S. Long, A. Kamaruddin and S. Bhatia, J. Membr. Sci., 247, 185 (2005).
A. Papra, H.G. Hicke and D. Paul, J. Appl. Polym. Sci., 74, 1669 (1999).
Y. Ito, Y. Ochiai, Y. S. Park and Y. Imanishi, J. Am. Chem. Soc., 119, 1619 (1997).
J. Yang and D. S. Hage, J. Chromatogr., 645, 241 (1993).
T. Kimura, K. Nakanishi, T. Nakagawa, A. Shibukawa and K. Matsuzaki, Pharm. Res., 22, 667 (2005).
C. L. Su, R. J. Dai, B. Tong and Y. L. Deng, Chinese Chem. Lett., 17, 649 (2006).
W. R. Bowen and R. R. Nigmatullin, Sep. Sci. Technol., 37, 3227 (2002).
T. Gumi, C. Minguillon and C. Palet, Polymer, 46, 12306 (2005).
A. Higuchi, H. Yomogita, B. O. Yoon, T. Kojima, M. Hara, S. Maniwa and M. Saitoh, J. Membr. Sci., 205, 203 (2002).
H.A. Wagenknecht, E.D. A. Stemp and J. K. Barton, J. Am. Chem. Soc., 122, 1 (2000).
A. Higuchi, Y. Ishida and T. Nakagawa, Desalination, 90, 127 (1993).
F. Garnier, J. Randon and J. L. Rocca, Sep. Purif. Technol., 16, 243 (1999).
S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 1, 14 (2004).
S. Tone, T. Masawaki and T. Hamada, J. Membr. Sci., 103, 57 (1995).
S. Tone, T. Masawaki and K. Eguchi, J. Membr. Sci., 118, 31 (1996).
T. Aoki, T. Fukuda, K. I. Shinohara, T. Kaneko, M. Teraguchi and M. Yagi, J. Polym. Sci., Polym. Chem., 42, 4502 (2004).
A. Higuchi, A. Hayashi, N. Kanda, K. Sanui and H. Kitamura, J. Mol. Str., 739, 145 (2005).
Y. Matsuoka, N. Kanda, Y.M. Lee and A. Higuchi, J. Membr. Sci., 280, 116 (2006).
A. Higuchi, Y. Higuchi, K. Furuta, B. O. Yoon, M. Hara, S. Maniwa, M. Saitoh and K. Sanui, J. Membr. Sci., 221, 207 (2003).
I.-C. Kim, J. Jegal and K.-H. Lee, J. Polym. Sci., Part B: Polymer Physics, 40, 2151 (2002).
T. Gumi, M. Valiente and C. Palet, J. Membr. Sci., 256, 150 (2005).
J. Randon, F. Garnier, J. L. Rocca and B. Maisterrena, J. Membr. Sci., 175, 111 (2000).
S.B. Lee, D. T. Mitchell, L. Tron, T.K. Nevanen, H. Soderlund and C. R. Martin, Science, 296, 2198 (2002).
T. Aoki, K. Shinohara and E. Oikawa, Makromol. Chem. Rapid Commun., 13, 565 (1992).
M. Teraguchi and T. Masuda, Macromolecules, 35, 1149 (2002).
J. Paris, C. Molina-Jouve, D. Nuel, P. Moulin and F. Charbit, J. Membr. Sci., 237, 9 (2004).
M. Yoshikawa, K. Murakoshi, T. Kogita, K. Hanaoka, M.D. Guiver and G. P. Robertson, Europ. Polym. J., 42, 2532 (2006).
M. Nakagawa, Y. Ikeuchi and M. Yoshikawa, Polymer, 49, 4612 (2008).
M. Yoshikawa, Y. Kondo and Y. Morita, Bioseparation, 10, 323 (2001).
P.G. Ingole, K. Singh and H.C. Bajaj, Arab. J. Chem. (2011), DOI: 10.1016/j.arabjc.2011.10.011.
M. Yoshikawa, T. Ooi and J.-I. Izumi, Eur. Polym. J., 37, 335 (2001).
J.T. F. Keurentjes, L. J.W.M. Nabuurs and E.A. Vegter, J. Membr. Sci., 113, 351 (1996).
M. Ulbricht, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 804, 113 (2004).
E.M. Van der Ent, K. Van’t Riet, J. T. F. Keurentjes and A. Van der Padt, J. Membr. Sci., 185, 207 (2001).
B. B. Lakshmi and C. R. Martin, Nature, 388, 758 (1997).
K.B. Jirage and C.R. Martin, Trends Biotechnol., 17, 197 (1999).
P.G. Ingole, W. Choi, K. H. Kim, H. D. Jo, W. K. Choi, J. S. Park and H. K. Lee, Desalination, 345, 136 (2014).
P.G. Ingole, W. Choi, K. H. Kim, C. H. Park, W.K. Choi and H. K. Lee, Chem. Eng. J., 243, 137 (2014).
A. Prakash Rao, S.V. Joshi, J. J. Trivedi, C.V. Devmurari and V. J. Shah, J. Membr. Sci., 211, 13 (2003).
S. Leob and S. Sourirajan, Reverse Osmosis, National Research Council of Canada, Ottawa, Canada (1985).
S.H. Yun, P.G. Ingole, K. H. Kim, W. K. Choi, J.H. Kim and H.K. Lee, Chem. Eng. J., 258, 348 (2014).
J. E. Cadotte, R. J. Petersen, R. E. Larson and E. E. Erickson, Desalination, 32, 25 (1980).
K. Singh, H.C. Bajaj, P. Ingole and A. Bhattacharya, Sep. Sci. Technol., 45, 346 (2010).
K. Singh, H. C. Bajaj and P. G. Ingole, Patent Number(s): WO2010109490-A1; IN200900629-I1 (2010).
P.G. Ingole, K. Singh and H. C. Bajaj, Sep. Sci. Technol., 46, 1898 (2011).
K. Singh, P.G. Ingole, J. Chaudhary, H. Bhrambhatt, A. Bhattacharya and H. C. Bajaj, J. Membr. Sci., 378, 531 (2011).
P.G. Ingole, K. Singh and H. C. Bajaj, Ind. J. Chem. Technol., 18, 197 (2011).
P.G. Ingole, K. Singh and H.C. Bajaj, J. Trends Chem., 1, 1 (2010).
K. Singh, P. G Ingole, H. Bhrambhatt, A. Bhattacharya and H. C. Bajaj, Sep. Purif. Technol., 78, 138 (2011).
P. G. Ingole, K. Singh and H. C. Bajaj, Desalination, 281, 413 (2011).
P.G. Ingole, H.C. Bajaj and K. Singh, Desalination, 305, 54 (2012).
P.G. Ingole, H.C. Bajaj and K. Singh, Desalination, 343, 75 (2014).
P.G. Ingole, H. C. Bajaj and K. Singh, Procedia Engineering, 44, 358 (2012).
P. G. Ingole, H. C. Bajaj and K. Singh, RSC Advances, 3, 3667 (2013).
K. Singh, P.G. Ingole, H.C. Bajaj and H. Gupta, Desalination, 298, 13 (2012).
P.G. Ingole, K. Singh, H.C. Bajaj, D.N. Srivastava and B. Rebary, Sep. Sci. Technol., 48, 1777 (2013).
P.G. Ingole, K. Singh and H.C. Bajaj, Adv. Mater. Res., 2, 1 (2013).
K. Singh, H. C. Bajaj and P. G. Ingole, Patent Number(s): WO2013118148-A1; PCT/IN2013/000081 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingole, P.G., Ingole, N.P. Methods for separation of organic and pharmaceutical compounds by different polymer materials. Korean J. Chem. Eng. 31, 2109–2123 (2014). https://doi.org/10.1007/s11814-014-0284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0284-z