Abstract
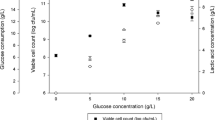
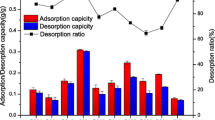
Precipitation is a simple, efficient method for separating and recovering lactic acid in the form of calcium lactate from fermentation broth by adding sulfuric acid. Major operating parameters of the recovery step as well as the temperature of concentration of the recovered lactic acid solution and the type and amount of adsorbent used for pigment (color) removal were optimized. When the molar ratio of calcium lactate to sulfuric acid was 1: 1 and the pH was increased to a value greater than the pKa (3.86), calcium sulfate was precipitated and could be removed more effectively, allowing for more efficient separation and recovery of supernatant lactic acid. Precipitation could be facilitated by adding calcium lactate solution with mixing (up to 220 rpm) and was completed in over 18 h. The optimal temperature for the concentration of lactic acid recovered from the supernatant after removing the precipitated calcium sulfate was found to be 90 °C in terms of the time required for concentration and the stability of the product. Activated carbon (SX-PLUS, 9 g/L) was most effective as an adsorbent for color removal from the recovered lactic acid. Under the optimized precipitation conditions, an overall yield of 92% of lactic acid from fermentation broth could be achieved.
Similar content being viewed by others
References
B. H. Lunelli, R. R. Andrade, D. I. Atala, M. R. Wolf Maciel, F. Maugeri Filho and R. Maciel Filho, Appl. Biochem. Biotechnol., 161, 227 (2010).
M. Sauer, D. Porro, D. Mattanovich and P. Branduardi, Trends Biotechnol., 26, 100 (2008).
B. Zhao, L. Wang, F. Li, D. Hua, C. Ma, Y. Ma and P. Xu, Bioresour. Technol., 101, 6499 (2010).
Z. Li, S. Ding, Z. Li and T. Tan, Biotechnol. J., 1, 1453 (2006).
M. K. H. Liew, S. Tanaka and M. Morita, Desalination, 101, 269 (1995).
W. Zhao, X. Sun, Q. Wang, H. Ma and Y. Teng, Biomass Bioenerg., 33, 21 (2009).
E.G. Lee, S. H. Kang, H. H. Kim and Y. K. Chang, Biotechnol. Bioprocess Eng., 11, 313 (2006).
K.-L. Ho, A. L. Pometto III and P. N. Hinz, Appl. Environ. Microbiol., 63, 2533 (1997).
K. L. Wasewar, A. B. M. Heesink, G. F. Versteeg and V. G. Pangarkar, J. Chem. Technol. Biotechnol., 77, 1068 (2002).
Y. Seo, W. H. Hong and T.H. Hong, Korean J. Chem. Eng., 16, 556 (1999).
M. Marinova, G. Kyuchoukov, J. Albet, J. Molinier and G. Malmary, Sep. Purif. Technol., 37, 199 (2004).
S. S. Yi, Y. C. Lu and G. S. Luo, Sep. Purif. Technol., 60, 308 (2008).
S. A. Ataei and E. Vasheghani-Farahani, J. Ind. Microbiol. Biotechnol., 35, 1229 (2008).
S. C. Park, S. M. Lee, Y. J. Kim, W. S. Kim and Y. M. Koo, Korean J. Biotechnol. Bioeng., 21, 199 (2006).
E. N. Kaufman, S. P. Cooper, S. L. Clement and M. H. Little, Appl. Biochem. Biotechnol., 45/46, 605 (1995).
M. Joseph, A. Eyal, C. Riki, B. Hazan and N. J. Starr, US Patent, 7,026,145 B2 (2006).
M. I. González, S. Álvarez, F. A. Riera and R. Álvarez, Ind. Eng. Chem. Res., 45, 3243 (2006).
W. S. Kim and E.K. Lee, Korean J. Biotechnol. Bioeng., 20, 164 (2005).
D. H. Lee, S.G. Kim, S. Mun and J. H. Kim, Process Biochem., 45, 1134 (2010).
S. H. Pyo H. B. Park, B. K. Song, B. H. Han and J. H. Kim, Process Biochem., 39, 1985 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, DJ., Choi, K.H., Chang, Y.K. et al. Effect of operating parameters on precipitation for recovery of lactic acid from calcium lactate fermentation broth. Korean J. Chem. Eng. 28, 1969–1974 (2011). https://doi.org/10.1007/s11814-011-0082-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0082-9