Abstract
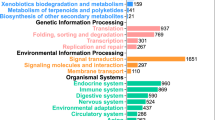
In this study, juvenile turbot Scophthalmus maximus were vaccinated with attenuated Edwardsiella tarda (EIBAV1) and reared at two different densities, low density (LD), (5.25±0.02)kgm−2, as control group and high density (HD), (20.53±0.05) kg m−2, as experimental group. Only density was considered as the variable. Five weeks after vaccination, the transcriptomes of spleen and head kidney from the turbot in two groups were analyzed with RNA-Seq technology. A total of 447 million reads were assembled into 41136 genes with an average length of 1274 bp and a N50 size of 2295 bp. A comparison of gene expression between HD and LD groups revealed 1155 differentially expressed genes (DEGs). Enrichment and pathway analysis of the 10 immune-related DEGs showed the centrality of toll-like receptor signaling pathway, cytosolic DNA-sensing pathway and platelet activation in the host immune responses. The 5 overexpressed inflammatory cytokines and 5 downregulated signal-regulated cytokines genes are covered by these immune-related DEGs. It was inferred that cells suffer damage and the immune response is restrained in turbot under crowding stress.
Similar content being viewed by others
References
Antonio, F., Diego, R., André, C., Miguel, H., Patricia, P., Juan, R., Jèssica, G. G., Laia, C., Xabier, B., Marta, G., Marina, M. H., Gabriel, F. C., Beatriz, G., José, L. G., José, L. A. F., Belen, G. P., Xoana, T., Carlos, F., Anna, V., Antonio, H. P., Roderic, G., José, A. Á. D., Antonio, G. T., Ana, V., Xulio, M., Toni, G., Beatriz, N., Carmen, B., Tyler, A., and Paullino, M., 2016. Whole genome sequencing of turbot (Scophthalmus maximus; Pleuronectiformes): A fish adapted to demersal life. DNA Research, 23(3): 181–192.
Aslam, R., Speck, E. R., Kim, M., Andrew, R. C., Bang, K. W. A., Nestel, F. P., Ni, H., Lazarus, A. H., Freedman, J., and Semple, J. W., 2006. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood, 107(2): 637–641.
Bagasra, O., 1997. The immunobiology of interferon-gamma inducible protein 10kD (IP-10): A novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine & Growth Factor Reviews, 8(3): 207–219.
Bhattacharjee, R., Xiang, W., Wang, Y., Zhang, X. Y., and Billiar, T. R., 2012. cAMP prevents TNF-induced apoptosis through inhibiting DISC complex formation in rat hepatocytes. Biochemical and Biophysical Research Communications, 423(1): 85–90.
Campbell, M. A., Chen, W. J., and López, J. A., 2014. Molecular data do not provide unambiguous support for the monophyly of flatfishes (Pleuronectiformes): A reply to Betancur-R and Ortí. Molecular Phylogenetics & Evolution, 75(1): 149–153.
Chen, S. L., Liu, Y., Dong, X. L., and Meng, L., 2010. Cloning, characterization, and expression analysis of a CC chemokine gene from turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 36(2): 147–155.
Chen, S., Zhang, G., Shao, C., Huang, Q., Liu, G., Zhang, P., Song, W., An, N., Chalopin, D., Volff, J. N., Hong, Y., Li, Q., Sha, Z., Zhou, H., Xie, M., Yu, Q., Liu, Y., Xiang, H., Wang, N., Wu, K., Yang, C., Zhou, Q., Liao, X., Yang, L., Hu, Q., Zhang, J., Zhu, Y., Du, M., Zhao, Y., Schartl, M., Tang, Q., and Wang, J., 2014. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics, 46(3): 253.
Cognasse, F., Hamzeh, H., Chavarin, P., Acquart, S., Genin, C., and Garraud, O., 2005. Evidence of Toll-like receptor molecules on human platelets. Immunology & Cell Biology, 83(2): 196–198.
Coppinger, J. A., Cagney, G., Toomey, S., Kislinger, T., Belton, O., Mcredmond, J. P., Cahill, D. J., Emili, A., Fitzgerald, D. J., and Maguire, P. B., 2004. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood, 103(6): 2096–2104.
Cox, D., Kerrigan, S. W., and Watson, S. P., 2011. Platelets and the innate immune system: Mechanisms of bacterial-induced platelet activation. Journal of Thrombosis & Haemostasis, 9(6): 1097.
Cristea, V., Mocanu, M., Antache, A., Docan, A., Dediu, L., Ion, Sandita., and Coadă, M. T., 2012. Effect of stocking density on leuckocyte reaction of Oncorhynchus mykiss (Walbaum, 1792). Lucrari Stiintifice Zootehnie Si Biotehnologii, 45(2): 31–36.
Cruciat, C. M., Dolde, C., de Groot, R. E., Ohkawara, B., Reinhard, C., Korswaqen, H. C., and Niehrs, C., 2013. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in wnt-β-catenin signaling. Science, 339(6126): 1436–1441.
de Souza, A. L., and Seguro, A. C., 2008. Two centuries of meningococcal infection: From vieusseux to the cellular and molecular basis of disease. Journal of Medical Microbiology, 57(11): 1313–1321.
Dou, F., Yuan, L. D., and Zhu, J. J., 2005. Heat shock protein 90 indirectly regulates ERK activity by affecting RAF protein metabolism. Acta Biochimica et Biophysica Sinica, 37(7): 501–505.
Du, X., Li, Y., Li, D., Lian, F., Yang, S., Wu, J., Liu, H., Bu, G., Meng, F., Cao, X., Zeng, X., Zhang, H., and Chen, Z., 2017. Transcriptome profiling of spleen provides insights into the antiviral mechanism in Schizothorax prenanti, after poly (i:c) challenge. Fish & Shellfish Immunology, 62: 13–23.
Dulay, A. T., Buhimschi, C. S., Zhao, G., Oliver, E. A., Mbele, A., Jing, S. C., and Buhimschi, I. A., 2009. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. Journal of Immunology, 182(11): 7244–7253.
Ellis, A. E., 2001. Innate host defense mechanisms of fish against viruses and bacteria. Developmental & Comparative Immunology, 25(8): 827–839.
Fitzgerald, J. R., Foster, T. J., and Cox, D., 2006. The interaction of bacterial pathogens with platelets. Nature Reviews Microbiology, 4(6): 445–457.
Fritz, J. H., Brunner, S., Birnstiel, M. L., Buschle, M., Gabain, A., Mattner, F., and Zauner, W., 2004. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine, 22(25–26): 3274–3284.
Fullam, A., and Schröder, M., 2013. DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication. Biochimica et Biophysica Acta, 1829(8): 854–865.
Gao, C., Qiang, F., Su, B., Zhou, S., Liu, F., Song, L., Zhang, M., Ren, Y., Dong, X., Tan, F., and Li, C., 2016. Transcriptomic profiling revealed the signatures of intestinal barrier alteration and pathogen entry in turbot (Scophthalmus maximus) following Vibrio anguillarum, challenge. Developmental & Comparative Immunology, 65: 159–168.
Garraud, O., and Cognasse, F., 2010. Platelet toll-like receptor expression: The link between ‘danger’ ligands and inflammation. Inflammation & Allergy Drug Targets, 9(5): 322–333.
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., Adiconis, X., Fan, L., Raychowdhury, R., Zeng, Q., Chen, Z., Mauceli, E., Hacohen, N., Gnirke, A., Rhind, N., Palma, F. D., Birren, B. W., Nusbaum, C., Toh, K. L., Friedman, N., and Regev, A., 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology, 29(7): 644–652.
Grandoch, M., Roscioni, S. S., and Schmidt, M., 2010. The role of epac proteins, novel camp mediators, in the regulation of immune, lung and neuronal function. British Journal of Pharmacology, 159(2): 265–284.
Griffiths, E. K., Krawczyk, C., Kong, Y. Y., Raab, M., Hyduk, S. J., Bouchard, D., Chan, V. S., Kozieradzki, I., Oliveira-Dos-Santos, A. J., Wakeham, A., Ohashi, P. S., Cybulsky, M. I., Rudd, C. E., and Penninger, J. M., 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science, 293(5538): 2260–2263.
Han, J., Lim, C. J., Watanabe, N., Soriani, A., Ratnikow, B., Calderwood, D. A., Puzon-Mclaughlin, W., Lafuente, E. M., Boussiotis, V. A., Shattil, S. J., and Ginsberg, M. H., 2006. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Current Biology, 16(18): 1796–1806.
Helmreich, E. J. M., 2001. Components of Signaling Networks: Linkers and Regulators. The Biochemistry of Cell Signaling. Oxford University Press, New York, 368pp.
Huo, H., Yin, S., Jia, R., Huang, B., Lei, J., and Liu, B., 2017. Effect of crowding stress on the immune response in turbot (Scophthalmus maximus) vaccinated with attenuated Edwardsiella tarda. Fish & Shellfish Immunology, 67(5): 353–358.
Iguchi, K., Ogawa, K., Nagae, M., and Ito, F., 2003. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture, 220(1): 515–523.
Ikeyama, S., Kokkonen, G., Shack, S., Wang, X. T., and Holbrook, N. J., 2002. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. Faseb Journal, 16(1): 114–116.
Jia, R., Liu, B. L., Feng, W. R., Han, C., Huang, B., and Lei, J. L., 2016. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish & Shellfish Immunology, 55: 131–139.
Juell, J. E., and Fosseidengen, J. E., 2004. Use of artificial light to control swimming depth and fish density of Atlantic salmon (Salmo salar) in production cages. Aquaculture, 233(1–4): 269–282.
Li, G., Zhao, Y., Liu, Z., Gao, C., Yan, F., Liu, B., and Feng, J., 2015. De novo assembly and characterization of the spleen transcriptome of common carp (Cyprinus carpio) using illumina paired-end sequencing. Fish & Shellfish Immunology, 44(2): 420–429.
Li, R., Yu, C., Li, Y., Lam, T., Yiu, S., Kristiansen, K., and Wang, J., 2009. Soap2: An improved ultrafast tool for short read alignment. Bioinformatics, 25(15): 1966–1967.
Liang, Q., Seo, G. J., Choi, Y. J., Kwak, M., Ge, J., Rodgers, M. A., Shi, M., Leslie, B. J., Hopfner, K., Ha, T., Oh, B., and Jung, J. U., 2014. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host & Microbe, 15(2): 228–238.
Liu, G., Zhang, L., and Zhao, Y., 2010. Modulation of immune responses through direct activation of toll-like receptors to T cells. Clinical & Experimental Immunology, 160(2): 168–175.
Liu, Y., Chen, S. L., Meng, L., and Zhang, Y. X., 2007. Cloning, characterization and expression analysis of a novel CXC chemokine from turbot (Scophthalmus maximus). Fish & Shellfish Immunology, 23(4): 711–720.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4): 402–408.
Long, Y., Li, Q., Zhou, B., Song, G., Li, T., and Cui, Z., 2013. De novo assembly of mud loach (Misgurnus anguillicaudatus) skin transcriptome to identify putative genes involved in immunity and epidermal mucus secretion. PLoS One, 8(2): e56 998.
Marshall, N. A., Galvin, K. C., Corcoran, A. M., Boon, L., Hiqqs, R., and Mills, K. H., 2012. Immunotherapy with PI3K inhibitor and toll-like receptor agonist induces IFN-γ+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Research, 72(3): 581–591.
Mortazavi, A., Williams, B. A., Mccue, K., Schaeffer, L., and Wold, B., 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods, 5(7): 621–628.
Nakanishi, K., Yoshimoto, T., Tsutsui, H., and Okamura, H., 2001. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine & Growth Factor Reviews, 12(1): 53–72.
Nardocci, G., Navarro, C., Cortés, P. P., Imarai, M., Montoya, M., Valenzuela, B., Jara, P., Castillo, C. A., and Fernánde, R., 2014. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish & Shellfish Immunology, 40(2): 531–538.
Neiva, B., Ronaldolima, D. L., Bernardo, B., Alcirluiz, D., and Alexpires, D. O. N., 2010. Growth, biochemical and physiological responses of Salminus brasiliensis with different stocking densities and handling. Aquaculture, 301(1–4): 22–30.
North, B. P., Turnbull, J. F., Ellis, T., Port, M. J., Migaud, H., Bron, J., and Bromage, N. R., 2006. The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture, 255(1–4): 466–479.
Oda, S., Schröder, M., and Khan, A. R., 2009. Structural basis for targeting of human RNA helicase DDX3 by poxvirus protein K7. Structure, 17(11): 1528–1537.
Padmini, E., and Tharani, J., 2014. Fourier transform infrared spectroscopic study on HSP70 and ERK in fish hepatocytes during pollution induced stress. International Journal of Research in Chemistry and Environment, 4(2): 114–125.
Pasare, C., and Medzhitov, R., 2004. Toll-like receptors: Linking innate and adaptive immunity. Microbes & Infection, 6(15): 1382–1387.
Patricia, P., Pablo, B., Alejandro, R., Sonia, D., Gabriel, F., Berta, F., Josep, V. P., Sergi, B., Beatriz, N., and Antonio, F., 2012. High-throughput sequence analysis of turbot (Scophthalmus maximus) transcriptome using 454-pyrosequencing for the discovery of antiviral immune genes. PLoS One, 7(5): e35369.
Peatman, E., Li, C., Peterson, B. C., Straus, D. L., Farmer, B. D., and Beck, B. H., 2013. Basal polarization of the mucosal compartment in Flavobacterium columnare susceptible and resistant channel catfish (Ictalurus punctatus). Molecular Immunology, 56(4): 317–327.
Pulsford, A. L., Crampe, M., Langston, A., and Glynn, P. J., 1995. Modulatory effects of disease, stress, copper, TBT and vitamin E on the immune system of flatfish. Fish & Shellfish Immunology, 5(8): 631–643.
Rehani, K., Wang, H., Garcia, C. A., Kinane, D. F., and Martin, M., 2009. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. Journal of Immunology, 182(1): 547–553.
Rengaraj, D., Truong, A. D., Lee, S. H., Lillehoj, H. S., and Hong, Y. H., 2016. Expression analysis of cytosolic DNA-sensing pathway genes in the intestinal mucosal layer of necrotic enteritis-induced chicken. Veterinary Immunology & Immunopathology, 170: 1–12.
Robledo, D., Hermida, M., Rubiolo, J. A., Fernández, C., Blanco, A., Bouza, C., and Martínez, P., 2016. Integrating genomic resources of flatfish (Pleuronectiformes) to boost aquaculture production. Comparative Biochemistry & Physiology Part D Genomics & Proteomics, 21: 41–55.
Robledo, D., Ronza, P., Harrison, P. W., Losada, A. P., Bermúdez, R., Pardo, B. G., Redondo, M. J., Bobadilla, A. S., Quiroga, M. I., and Martínez, P., 2014. RNA-Seq analysis reveals significant transcriptome changes in turbot (Scophthalmus maximus) suffering severe enteromyxosis. BMC Genomics, 15(1): 1149.
Roers, A., Hiller, B., and Hornung, V., 2016. Recognition of endogenous nucleic acids by the innate immune system. Immunity, 44(4): 739–754.
Ronza, P., Robledo, D., Bermúdez, R., Losada, A. P., Pardo, B. G., Bobadilla, A. S., Quiroga, M. I., and Martínez, P., 2016. RNA-Seq analysis of early enteromyxosis in turbot (Scophthalmus maximus): New insights into parasite invasion and immune evasion strategies. International Journal for Parasitology, 46(8): 507–517.
Schneider, W. M., Chevillotte, M. D., and Rice, C. M., 2014. Interferon-stimulated genes: A complex web of host defenses. Annual Review of Immunology, 32(1): 513–545.
Shi, Z., Hodges, V. M., Dunlop, E. A., Percy, M. J., Maxwell, A. P., EI-Tanani, M., and Lappin, T. R., 2010. Erythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell line. Molecular Cancer Research, 8(4): 615–626.
Thompson, M. R., Kaminski, J. J., Kurtjones, E. A., and Fitzgerald, K. A., 2011. Pattern recognition receptors and the innate immune response to viral infection. Viruses, 3(6): 920–940.
Tort, L., 2011. Stress and immune modulation in fish. Developmental & Comparative Immunology, 35(12): 1366–1375.
Turnbull, J., Bell, A., Adams, C., Bron, J., and Huntingford, F., 2005. Stocking density and welfare of cage farmed atlantic salmon: Application of a multivariate analysis. Aquaculture, 243(1–4): 121–132.
Vargas-Chacoff, L., Martínez, D., Oyarzún, R., Nualart, D., Olavarría, V., Yáñez, A., Bertrán, C., Ruiz-Jarabo, I., and Mancera, J. M., 2014. Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the sub-antarctic notothenioid fish Eleginops maclovinus. Fish & Shellfish Immunology, 40(2): 424–434.
Verburg-van Kemenade, B. L., Nowak, B., Engelsma, M. Y., and Weyts, F. A. A., 1999. Differential effects of cortisol on apoptosis and proliferation of carp B-lymphocytes from head kidney, spleen and blood. Fish & Shellfish Immunology, 9(5): 405–415.
Watson, R., Bell, S., Macduff, D., Kimmey, J., Diner, E., Olivas, J., Vance, R., Stallings, C., Virgin, H., and Cox, J., 2015. The cytosolic sensor cGAS detects Mycobacterium tuberculosis, DNA to induce type I interferons and activate autophagy. Cell Host & Microbe, 17(6): 811.
Werling, D., Jann, O. C., Offord, V., Glass, E. J., and Coffey, T. J., 2009. Variation matters: TLR structure and species-specific pathogen recognition. Trends in Immunology, 30(3): 124–130.
Wu, J., and Chen, Z. J., 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annual Review of Immunology, 32(1): 461.
Xiang, L. X., He, D., Dong, W. R., Zhang, Y. W., and Shao, J. Z., 2010. Deep sequencing-based transcriptome profiling analysis of bacteria-challenged Lateolabrax japonicus reveals insight into the immune-relevant genes in marine fish. BMC Genomics, 11(472): 1–21.
Xiao, J., Chen, T., Liu, B., Yang, W., Wang, Q., Qu, J., and Zhang, Y., 2013. Edwardsiella tarda mutant disrupted in type III secretion system and chorismic acid synthesis and cured of a plasmid as a live attenuated vaccine in turbot. Fish & Shellfish Immunology, 35(3): 632–641.
Yang, K., Wang, J., and Wu, M., 2015. Mesenchymal stem cells detect and defend against gammaherpesvirus infection via the cGAS-STING pathway. Scientific Reports, 5(7820): 1–9.
Yarahmadi, P., Miandare, H. K., Hoseinifar, S. H., Gheysvandi, N., and Akbarzadeh, A., 2015. The effects of stocking density on hemato-immunological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquaculture International, 23(1): 55–63.
Yeaman, M. R., 2010. Bacterial-platelet interactions: Virulence meets host defense. Future Microbiology, 5(3): 471–506.
Yeaman, M. R., Bayer, A. S., Koo, S. P., Foss, W., and Sullam, P. M., 1998. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. Journal of Clinical Investigation, 101(1): 178–187.
Yin, F., Gao, Q., Tang, B., Sun, P., Han, K., and Huang, W., 2016. Transcriptome and analysis on the complement and coagulation cascades pathway of large yellow croaker (Larimichthys crocea) to ciliate ectoparasite Cryptocaryon irritans infection. Fish & Shellfish Immunology, 50: 127–141.
Yoshie, O., Imai, T., and Nomiyama, H., 2001. Chemokines in immunity. Advances in Immunology, 78(78): 57–110.
Zebral, Y. D., Zafalon-Silva, B., Mascarenhas, M. W., and Robaldo, R. B., 2015. Leucocyte profile and growth rates as indicators of crowding stress in pejerrey fingerlings (Odontesthes bonariensis). Aquaculture Research, 46(9): 2270–2276.
Zhang, X., Wang, S., Chen, S., Chen, Y., Liu, Y., Shao, C., Wang, Q., Lu, Y., Gong, G., Ding, S., and Sha, Z., 2015. Transcriptome analysis revealed changes of multiple genes involved in immunity in Cynoglossus semilaevis during Vibrio anguillarum infection. Fish & Shellfish Immunology, 43(1): 209–218.
Zhu, J., Li, C., Ao, Q., Tan, Y., Luo, Y., Guo, Y., Lan, G., Jiang, H., and Gan, X., 2015. Trancriptomic profiling revealed the signatures of acute immune response in tilapia (Oreochromis niloticus), following Streptococcus iniae, challenge. Fish & Shellfish Immunology, 46(2): 346–353.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2017YFB0404001), the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (No. 2017HYZD04), the Qingdao Shinan District Science and Technology Bureau (No. 2016-3-006) and the Modern Agriculture Industry System Construction Special Funds (No. CARS-47-G24).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, H., Gao, X., Fei, F. et al. Transcriptomic Profiling of the Immune Response to Crowding Stress in Juvenile Turbot (Scophthalmus maximus). J. Ocean Univ. China 19, 911–922 (2020). https://doi.org/10.1007/s11802-020-4242-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-020-4242-6