Abstract
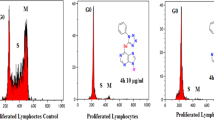
Cytarabine (1-β-D-arabinofuranosylcytosine, Ara-C), isolated from a Caribbean sponge species Tethyacrypta, is the first antitumor drug from a marine resource. In 1980, the US Food and Drug Administration approved this drug for the treatment of different types of leukemia. This drug has a short plasma half-life, low stability, limited bioavailability, and severe side effects. To improve stability and bioavailability, we synthesized nine novel derivatives by blocking the cytarabine metabolic sites and improving lipophilicity. The cLogP values of the newly synthesized compounds were calculated. All the synthesized compounds were more lipophilic than cytarabine, resulting in membrane permeability and bioavailability improvement. The antitumor activities against leukemia cell line HL-60 were evaluated by using the MTT assay. The bioassay results revealed that the IC50 values of compounds 5, 8 and 9 were 0.080, 0.090 and 0.057 μmol L−1, respectively, which was similar with that of cytarabine (0.056 μmol L−1). In comparison, compound 4 with a phosphate group at O5’ was inactive. Because phosphoester bonds are easily hydrolyzed by alkaline phosphatase and are commonly used in producing prodrug strategies, compound 4 might also be metabolized in vivo and generate compound 3 or even cytarabine through a multi-step reaction. Thus, compound 4 might be a promising compound to be developed as a prodrug.
Similar content being viewed by others
References
Adema, A. D., Losekoot, N., Smid, K., Kathmann, I., Myhren, F., Sandvold, M. L., and Peters, G. J., 2010. Induction of resistance to the lipophilic cytarabine prodrug elacytarabine (CP-4055) in CEM leukemic cells. Nucleosides Nucleotides Nucleic Acids, 29: 394–399.
Braess, J., Pförtner, J., Kaufmann, C., Ramsauer, B., Unterhalt, M., Hiddemann, W., and Schleyer, E., 1996. Detection and determination of the major metabolites of [3 H] cytosine arabinoside by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 676: 131–140.
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., Jemal, A., Yu, X. Q., and He, J., 2016. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians, 66: 115–132.
Chhikara, B. S., and Parang, K., 2010. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opinion on Drug Delivery, 7: 1399–1414.
Cottam, H. B., and Carson, D. A., 2007. 2-chlorodeoxyadenosine (cladribine): Rational development of a novel chemotherapeutic agent. Cheminform, 38: 393–407.
DiNardo, C. D., O’Brien, S, Gandhi, V. V., and Ravandi, F., 2013. Elacytarabine (CP-4055) in the treatment of acute myeloid leukemia. Future Oncology, 9: 1073–1082.
Fadl, T. A., Hasegawa, T., Youssef, A. F., Farag, H. H., Omar, F. A., and Kawaguchi, T., 1995. Synthesis and investigation of N4-substituted cytarabine derivatives as prodrugs. Pharmazie, 50: 382–387.
Flores-Ramos, M., Ibarra-Velarde, F., Jung-Cook, H., Hernandez-Campos, A., Vera-Montenegro, Y., and Castillo, R., 2017. Novel triclabendazole prodrug: A highly water soluble alternative for the treatment of fasciolosis. Bioorganic Medicinal Chemistry Letters, 27: 616–619.
Gandhi, V., Estey, E., Keating, M. J., Chucrallah, A., and Plunkett, W., 1996. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: Pharmacokinetic, pharmacodynamic, and molecular interactions. Blood, 87: 256–264.
Hanson, B. A., Schowen, R. L., and Stella, V. J., 2003. A mechanistic and kinetic study of the E-ring hydrolysis and lactonization of a novel phosphoryloxymethyl prodrug of camptothecin. Pharmacological Research, 20: 1031–1038.
Liu, J., Zhao, D., He, W., Zhang, H., Li, Z., and Luan, Y., 2017. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. Journal of Colloid and Interface Science, 487: 239–249.
Maloisel, F., Guerci, A., Guyotat, D., Ifrah, N., Michallet, M., Reiffers, J., Tertain, G., Blanc, M., Bauduer, F., Briere, J., Abgrall, J. F., Pegourie-Bandelier, B., Solary, E., Cambier, N., Coso, D., Vilque, J. P., Delain, M., Harousseau, J. L., Rousselot, P., Belhadj, K., Morice, P., Attal, J., Chabin, M., Chastang, C., Guilhot, J., and Guilhot, F., 2002. Results of a phase II trial of a combination of oral cytarabine ocfosfate (YNK01) and interferon alpha-2b for the treatment of chronic myelogenous leukemia patients in chronic phase. Leukemia, 16: 573–580.
Montaser, R., and Luesch, H., 2011. Marine natural products: A new wave of drugs? Future Medicinal Chemistry, 3: 1475–1489.
Nam, N. H., Sardari, S., Selecky, M., and Parang, K., 2004. Carboxylic acid and phosphate ester derivatives of fluconazole: Synthesis and antifungal activities. Bioorganic Medicinal Chemistry, 12: 6255–6269.
Parker, E. N., Odutola, S. O., Wang, Y., Strecker, T. E., Mukherjee, R., Shi, Z., Chaplin, D. J., Trawick, M. L., and Pinney, K. G., 2016. Synthesis and biological evaluation of a watersoluble phosphate prodrug salt and structural analogues of KGP94, a lead inhibitor of cathepsin L. Bioorganic Medicinal Chemistry Letters, 27: 1304–1310
Pigneux, A., Perreau, V., Jourdan, E., Vey, N., Dastugue, N., Huguet, F., Sotto, J. J., Salmi, L. R., Ifrah, N., and Reiffers, J., 2007. Adding lomustine to idarubicin and cytarabine for induction chemotherapy in older patients with acute myeloid leukemia: The BGMT 95 trial results. Haematologica, 92: 1327–1334.
Sagar, S., Kaur, M., and Minneman, K. P., 2010. Antiviral lead compounds from marine sponges. Marine Drugs, 8: 2619–2638.
Siegel, R., Ward, E., Brawley, O., and Jemal, A., 2011. The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer Journal for Clinicians, 61: 212–236.
Sun, Y., Sun, J., Shi, S., Jing, Y., Yin, S., Chen, Y., Li, G., Xu, Y., and He, Z., 2008. Synthesis, transport and pharmacokinetics of 5’-amino acid ester prodrugs of 1-β-D-arabinofuranosylcytosine. Molecular Pharmaceutics, 6: 315–325.
Tamamyan, G., Kadia, T., Ravandi, F., Borthakur, G., Cortes, J., Jabbour, E., Daver, N., Ohanian, M., Kantarjian, H., and Konopleva, M., 2017. Frontline treatment of acute myeloid leukemia in adults. Critical Reviews in Oncology Hematology, 110: 20–34.
VandeVoorde, J., Vervaeke, P., Liekens, S., and Balzarini, J., 2015. Mycoplasma hyorhinis-encoded cytidinedeaminase efficiently inactivates cytosine-based anticancer drugs. FEBS Open Bio, 5: 634–639.
Woelich, S. K., Braun, J. T., Schoen, M. W., Ramlal, R., Freter, C. E., Petruska, P. J., and Lionberger, J. M., 2017. Efficacy and toxicity of induction therapy with cladribine, idarubicin, and cytarabine (IAC) for acute myeloid leukemia. Anticancer Research, 37: 713–717.
Acknowledgement
This study was supported by the ‘Zhufeng Scholar Program’ of Ocean University of China (No. 841412016), the ‘Outstanding Talents Plan’ of Qingdao (No. 15-10-3-15-(34)-zch) to Dr. Wenbao Li and youth special fund for PhD of Qingdao (No. 16-5-1-61-jch).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, Y., Tian, Z., Hou, Y. et al. Study on the Preparation and Antileukemic Activity of New Lipophilic 1-β-D-arabinofuranosylcytosine Derivatives. J. Ocean Univ. China 17, 385–391 (2018). https://doi.org/10.1007/s11802-018-3419-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-018-3419-8