Abstract
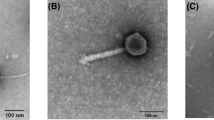
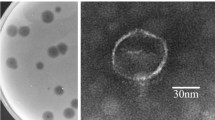
The bacteriophage P13 that infects Klebsiella serotype K13 contains a heat-stable depolymerase capable of effective degradation of exopolysaccharide (EPS) produced by this microorganism. In this study, the titer of phage P13, initially 2.0 × 107 pfu mL−1, was found increasing 20 min after infection and reached 5.0 × 109 pfu mL−1 in 60 min. Accordingly, the enzyme activity of depolymerase approached the maximum 60 min after infection. Treatment at 70°C for 30 min inactivated all the phage, but retained over 90% of the depolymerase activity. Addition of acetone into the crude phage lysate led to precipitation of the protein, with a marked increase in bacterial EPS degradation activity and a rapid drop in the titer of phage. After partial purification by acetone precipitation and ultrafiltration centrifugation, the enzyme was separated from the phage particles, showing two components with enzyme activity on Q-Sepharose Fast Flow. The soluble enzyme had an optimum degradation activity at 60°C and pH 6.5. Transmission electron microscopy demonstrated that the phage P13 particles were spherical with a diameter of 50 nm and a short stumpy tail. It was a double-strand DNA virus consisting of a nucleic acid molecule of 45976 bp. This work provides an efficient purification operation including thermal treatment and ultrafiltration centrifugation, to dissociate depolymerase from phage particles. The characterization of phage P13 and associated EPS depolymerase is beneficial for further application of this enzyme.
Similar content being viewed by others
References
Adams, M. H., and Park, B. H., 1956. An enzyme produced by a phage-host cell system. The properties of the polysacch II aride depolymerase. Virology, 2: 719–736.
Altmann, F., Christian, R., Czerny, T., Nimmich, W., and Marz, L., 1990. Bacteriophage-associated glycan hydrolases specific for Escherichia coli capsular serotype K12. European Journal of Biochemistry, 189: 307–312.
Bayer, M. E., and Anderson, T. F., 1963. The preparation of holey films for electron microscopy. Cellular and Molecular Life Sciences, 19: 433–434.
Cescutti, P., Toffanin, R., Kvam, B. J., Paoletti, S., and Dutton, G. G., 1993. Structural determination of the capsular polysaccharide produced by Klebsiella pneumoniae serotype K40. NMR studies of the oligosaccharide obtained upon depolymerisation of the polysaccharide with a bacteriophage-asso-ciated endoglycanase. European Journal of Biochemistry, 213: 445–453.
Coenye, T., and Nelis, H. J., 2010. In vitro and in vivo model systems to study microbial biofilm formation. Jounal of Microbiol Methods, 83: 89–105.
Davies, D., 2003. Understanding biofilm resistance to antibacterial agents. Nature Review Drug Discovery, 2: 114–122.
Di Fabio, J. L., Karunaratne, D. N., and Dutton, G. G., 1985. Novel oligosaccharides obtained by bacteriophage degradation of the polysaccharide from Klebsiella serotype K26. Carbohydrate Research, 144: 251–261.
Dutton, G. G. S., Di Fabio, J. L., Leek, D. M., Merrifield, E. H., Nunn, J. R., and Stephen, A. M., 1981. Preparation of oligosaccharides by the action of bacteriophage-borne enzymes on Klebsiella capsular polysaccharides. Carbohydrate Research, 97: 127–138.
Flint, S. H., Bremer, P. J., and Brooks, J. D., 1997. Biofilms in dairy manufacturing plant description, current concerns and methods of control. Biofouling, 11: 81–97.
Griffiths, A. J., and Davies, D. B., 1991. Type-specific carbohydrate antigens of pathogenic bacteria. Part 1: Enterobacteriaceae. Carbohydrate Polymers, 14: 241–279.
Habash, M., and Reid, G., 1999. Microbial biofilms: Their development and significance for medical device-related infections. The Journal of Clinical Pharmacology, 39: 887–898.
Hanlon, G. W., Denyer, S. P., Olliff, C. J., and Ibrahim, L. J., 2001. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology, 67: 2746–2753.
Hughes, K. A., Sutherland, I. W., and Jones, M. V., 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology, 144: 3039–3047.
Kassa, T., and Chhibber, S., 2012. Thermal treatment of the bacteriophage lysate of Klebsiella pneumoniae B5055 as a step for the purification of capsular depolymerase enzyme. Journal of Virological Methods, 179: 135–141.
Kim, W. S., and Geider, K., 2000. Characterization of a viral EPS-depolymerase, a potential tool for control of fire blight. Phytopathology, 90: 1263–1268.
King, A. M. Q., Adams, M. J., Carstens, E. B., and Lefkowitz, E. J., 2011. Virus taxonomy: ninth report of the international committee on taxonomy of viruses. In: Genus: SP6-Like Viruses. Elsevier Academic Press, San Diego, 75–76.
Mou, H., Wang, J., Jiang, X., and Liu, Z., 2008. Preparation and properties of bacteriophage-borne enzyme degrading bacterial exopolysaccharide. High Technology Letters, 14: 210–215.
Roberts, T. S., 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annual Review of Microbiology, 50: 285–315.
Scorpio, A., Tobery, S. A., Ribot, W. J., and Friedlander, A. M., 2008. Treatment of experimental anthrax with recombinant capsule depolymerase. Antimicrobial Agents and Chemotherapy, 52: 1014–1020.
Simoes, M., Simoes, L. C., and Vieira, M. J., 2010. A review of current and emergent biofilm control strategies. LWT-Food Science and Technology, 43: 573–583.
Stewart, P. S., Murga, R., and Srinivasan, R., 1995. Biofilm structural heterogeneity visualized by three microscopic methods. Water Research, 29: 2006–2009.
Sutherland, I. W., 1967. Phage-induced fucosidase hydrolysing the exopolysaccharide of Klebsiella aerogenes type 54. Biochemical Journal, 104: 278–285.
Sutherland, I. W., 2001. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology, 147: 3–9.
von Borel, E., Hostettler, F., and Deuel, H., 1952. Quantitative zuckerbestimmung mit 3, 5-dinitrosalicylsaure and phenol. Helvetica Chimica Acta, 35: 115–120.
Xu, G., and Chen, F., 2010. Biochemistry and technology practical training. In: Protein Content Detection by Coomassie Brilliant Blue Staining. Huazhong University of Science and Technology Press, Wuhan, 43–45.
Yurewicz, E. C., Ghalambor, M. A., Duckworth, D. H., and Heath, E. C., 1971. Catalytic and molecular properties of a phage-induced capsular polysaccharide depolymerse. Journal of Biological Chemistry, 246: 5607–5616.
Zhu, X., 2010. The experimental guide for gene engineering. In: Nucleic Acid Electrophoresis. Higner Education Press, Beijing, 74–77, 227–235.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Li, G., Mo, Z. et al. Properties of Klebsiella phage P13 and associated exopolysaccharide depolymerase. J. Ocean Univ. China 13, 163–168 (2014). https://doi.org/10.1007/s11802-014-2042-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-014-2042-6