Abstract
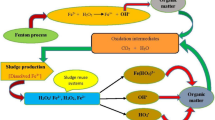
The classical Fenton oxidation process (CFOP) is a versatile and effective application that is generally applied for recalcitrant pollutant removal. However, excess iron sludge production largely restricts its widespread application. Fenton sludge is a hazardous solid waste, which is a complex heterogeneous mixture with Fe(OH)3, organic matter, heavy metals, microorganisms, sediment impurities, and moisture. Although studies have aimed to utilize specific Fenton sludge resources based on their iron-rich characteristics, few reports have fully reviewed the utilization of Fenton sludge. As such, this review details current sustainable Fenton sludge reuse systems that are applied during wastewater treatment. Specifically, coagulant preparation, the reuse of Fenton sludge as an iron source in the Fenton process and as a synthetic heterogeneous catalyst/adsorbent, as well as the application of the Fenton sludge reuse system as a heterogeneous catalyst for resource utilization. This is the first review article to comprehensively summarize the utilization of Fenton sludge. In addition, this review suggests future research ideas to enhance the cost-effectiveness, environmental sustainability, and large-scale feasibility of Fenton sludge applications.

Similar content being viewed by others
References
Ahmad M, Rajapaksha A U, Lim J E, Zhang M, Bolan N, Mohan D, Vithanage M, Lee S S, Ok Y S (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99: 19–33
Badawy M I, Ali M E (2006). Fenton’s peroxidation and coagulation processes for the treatment of combined industrial and domestic wastewater. Journal of Hazardous Materials, 136(3): 961–966
Barb W G, Baxendale J H, George P, Hargrave K R (1949). Reactions of ferrous and ferric ions with hydrogen peroxide. Nature, 163(4148): 692–694
Bautista P, Mohedano A F, Casas J A, Zazo J A, Rodriguez J J (2008). An overview of the application of Fenton oxidation to industrial wastewaters treatment. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 83(10): 1323–1338
Belete Y Z, Ziemann E, Gross A, Bernstein R (2021). Facile activation of sludge-based hydrochar by Fenton oxidation for ammonium adsorption in aqueous media. Chemosphere, 273: 128526
Bello M M, Abdul Raman A A, Asghar A (2019). A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Safety and Environmental Protection, 126: 119–140
Benatti C T, Costa A C, Tavares C R (2009). Characterization of solids originating from the Fenton’s process. Journal of Hazardous Materials, 163(2–3): 1246–1253
Benatti C T, Tavares C R, Guedes T A (2006). Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. Journal of Environmental Management, 80(1): 66–74
Bolobajev J, Kattel E, Viisimaa M, Goi A, Trapido M, Tenno T, Dulova N (2014). Reuse of ferric sludge as an iron source for the Fenton-based process in wastewater treatment. Chemical Engineering Journal, 255: 8–13
Bolobajev J, Trapido M, Goi A (2016a). Role of organic wastewater constituents in iron redox cycling for ferric sludge reuse in the fenton-based treatment. International Journal of Environmental and Ecological Engineering, 10: 39944
Bolobajev J, Trapido M, Goi A (2016b). Interaction of tannic acid with ferric iron to assist 2,4,6-trichlorophenol catalytic decomposition and reuse of ferric sludge as a source of iron catalyst in Fenton-based treatment. Applied Catalysis B: Environmental, 187: 75–82
Cao G M, Sheng M, Niu W F, Fei Y L, Li D (2009). Regeneration and reuse of iron catalyst for Fenton-like reactions. Journal of Hazardous Materials, 172(2–3): 1446–1449
Chen L, Ma J, Li X, Zhang J, Fang J, Guan Y, Xie P (2011). Strong enhancement on fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles. Environmental Science & Technology, 45(9): 3925–3930
Cheng M, Ma W, Li J, Huang Y, Zhao J, Wen Y, Xu Y (2004). Visible-light-assisted degradation of dye pollutants over Fe(III)-loaded resin in the presence of H2O2 at neutral pH values. Environmental Science & Technology, 38(5): 1569–1575
Chu J H, Kang J K, Park S, Lee C (2020). Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. Journal of Water Process Engineering. 37: 101455
Dantas E R B, Silva E J, Lopes W S, do Nascimento M R, Leite V D, de Sousa J T (2020). Fenton treatment of sanitary landfill leachate: Optimization of operational parameters, characterization of sludge and toxicology. Environmental Technology, 41(20): 2637–2647
De Laat J, Gallard H É (1999). Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: mechanism and kinetic modeling. Environmental Science & Technology, 33(16): 2726–2732
Demarchis L, Minella M, Nisticò R, Maurino V, Minero C, Vione D (2015). Photo-Fenton reaction in the presence of morphologically controlled hematite as iron source. Journal of Photochemistry and Photobiology A Chemistry, 307–308: 99–107
Deng Y, Englehardt J D (2006). Treatment of landfill leachate by the Fenton process. Water Research, 40(20): 3683–3694
Di Iaconi C, Del Moro G, De Sanctis M, Rossetti S (2010). A chemically enhanced biological process for lowering operative costs and solid residues of industrial recalcitrant wastewater treatment. Water Research, 44(12): 3635–3644
Diya’uddeen B H, Rahim Pouran S, Abdul Aziz A R, Daud W M (2015). Fenton oxidative treatment of petroleum refinery wastewater: process optimization and sludge characterization. RSC Advances, 5(83): 68159–68168
Duan X, Sun H, Shao Z, Wang S (2018). Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Applied Catalysis B: Environmental, 224: 973–982
Ensing B, Buda F, Baerends E J (2003). Fenton-like chemistry in water: Oxidation catalysis by Fe(III) and H2O2. Journal of Physical Chemistry A, 107(30): 5722–5731
Fan F (2016). The preparation of efficient magnetic polymeric ferric sulfate with recycled fenton iron sludge. Dissertation for the Master Degree. Guilin: Guangxi University (in Chinese)
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J, Wang Z, Li X (2016). Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. Journal of Molecular Liquids, 220: 432–441
Fang G, Gao J, Liu C, Dionysiou D D, Wang Y, Zhou D (2014). Key role of persistent free radicals in hydrogen peroxide activation by biochar: Implications to organic contaminant degradation. Environmental Science & Technology, 48(3): 1902–1910
Fernandez J, Bandara J, Kiwi J, Lopez A, Albers P (1998). Efficient photo-assisted Fenton catalysis mediated by Fe ions on Nafion membranes active in the abatement of non-biodegradable azo-dye. Chemical Communications, 14(14): 1493–1494
Gamaralalage D, Sawai O, Nunoura T (2017). Sludge reuse in Fenton oxidation of crepe rubber wastewater and palm oil mill effluent. In: The 28th Annual Conference of JSMCWM, 2017. Tokyo: JSMCWM, 5–6
Garade A C, Bharadwaj M, Bhagwat S V, Athawale A A, Rode C V (2009). An efficient γ-Fe2O3 catalyst for liquid phase air oxidation of p-hydroxybenzyl alcohol under mild conditions. Catalysis Communications, 10(5): 485–489
Ge J, Guha B, Lippincott L, Cach S, Wei J, Su T L, Meng X (2020). Challenges of arsenic removal from municipal wastewater by coagulation with ferric chloride and alum. Science of the Total Environment, 725: 138351
Ghernaout D, Elboughdiri N, Ghareba S (2020). Fenton technology for wastewater treatment: dares and trends. Open Access Library Journal, 7(01): 1–26
Guedes A M, Madeira L M, Boaventura R A, Costa C A (2003). Fenton oxidation of cork cooking wastewater-overall kinetic analysis. Water Research, 37(13): 3061–3069
Guo S, Yuan N, Zhang G, Yu J C (2017). Graphene modified iron sludge derived from homogeneous Fenton process as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants. Microporous and Mesoporous Materials, 238: 62–68
Guvenc S Y, Varank G (2021). Degradation of refractory organics in concentrated leachate by the Fenton process: Central composite design for process optimization. Frontiers of Environmental Science & Engineering, 15(1): 2
Hou X, Huang X, Jia F, Ai Z, Zhao J, Zhang L (2017). Hydroxylamine promoted goethite surface fenton degradation of organic pollutants. Environmental Science & Technology, 51(9): 5118–5126
Hua W (2017). Fenton wastewater treatment sludge disposal and recovery of iron salits using technology. Dissertation for the Master Degree. Guangzhou: South China University of Technology (in Chinese)
Ighalo J O, Adeniyi A G (2020). Adsorption of pollutants by plant bark derived adsorbents: An empirical review. Journal of Water Process Engineering, 35: 101228
Ince N H, Apikyan I G (2000). Combination of activated carbon adsorption with light-enhanced chemical oxidation via hydrogen peroxide. Water Research, 34(17): 4169–4176
Jain B, Singh A K, Kim H, Lichtfouse E, Sharma V K (2018). Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environmental Chemistry Letters, 16(3): 947–967
Kattel E, Trapido M, Dulova N (2016). Treatment of landfill leachate by continuously reused ferric oxyhydroxide sludge-activated hydrogen peroxide. Chemical Engineering Journal, 304: 646–654
Kavitha V, Palanivelu K (2004). The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere, 55(9): 1235–1243
Kishimoto N, Kitamura T, Kato M, Otsu H (2013). Reusability of iron sludge as an iron source for the electrochemical Fenton-type process using Fe2+/HOCl system. Water Research, 47(5): 1919–1927
Klein K, Kivi A, Dulova N, Zekker I, Molder E, Tenno T, Trapido M, Tenno T (2016). A pilot study of three-stage biological-chemical treatment of landfill leachate applying continuous ferric sludge reuse in Fenton-like process. Clean Technologies and Environmental Policy, 19(2): 541–551
Leifeld V, Dos Santos T P M, Zelinski D W, Igarashi-Mafra L (2018). Ferrous ions reused as catalysts in Fenton-like reactions for remediation of agro-food industrial wastewater. Journal of Environmental Management, 222: 284–292
Leng L, Yuan X, Huang H, Shao J, Wang H, Chen X, Zeng G (2015). Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption. Applied Surface Science, 346: 223–231
Li C W, Chen Y M, Chiou Y C, Liu C K (2007). Dye wastewater treated by Fenton process with ferrous ions electrolytically generated from iron-containing sludge. Journal of Hazardous Materials, 144(1–2): 570–576
Liao Q, Sun J, Gao L (2009). Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 345(1–3): 95–100
Lin S S, Gurol M D (1998). Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environmental Science & Technology, 32(10): 1417–1423
Mahiroglu A, Tarlan-Yel E, Sevimli M F (2009). Treatment of combined acid mine drainage (AMD—flotation circuit effluents from copper mine via Fenton’s process. Journal of Hazardous Materials, 166(2–3): 782–787
Mahtab M S, Farooqi I H, Khursheed A (2021a). Zero Fenton sludge discharge: A review on reuse approach during wastewater treatment by the advanced oxidation process. International Journal of Environmental Science and Technology, 10: 1–14
Mahtab M S, Islam D T, Farooqi I H (2021b). Optimization of the process variables for landfill leachate treatment using Fenton based advanced oxidation technique. Engineering Science and Technology, an International Journal, 24(2): 428–435
Meng H, Nie C, Li W, Duan X, Lai B, Ao Z, Wang S, An T (2020). Insight into the effect of lignocellulosic biomass source on the performance of biochar as persulfate activator for aqueous organic pollutants remediation: Epicarp and mesocarp of citrus peels as examples. Journal of Hazardous Materials, 399: 123043
Neyens E, Baeyens J (2003). A review of classic Fenton’s peroxidation as an advanced oxidation technique. Journal of Hazardous Materials, 98(1–3): 33–50
Oturan M A, Aaron J J (2014). Advanced oxidation processes in water/wastewater treatment: Principles and applications: A review. Critical Reviews in Environmental Science and Technology, 44(23): 2577–2641
Paciolla M D, Davies G, Jansen S A (1999). Generation of hydroxyl radicals from metal-loaded humic acids. Environmental Science & Technology, 33(11): 1814–1818
Pan X, Gu Z, Chen W, Li Q (2021). Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Science of the Total Environment, 754: 142104
Páramo-Vargas J, Granados S G, Maldonado-Rubio M I, Peralta-Hernández J M (2016). Up to 95% reduction of chemical oxygen demand of slaughterhouse effluents using Fenton and photo-Fenton oxidation. Environmental Chemistry Letters, 14(1): 149–154
Rossi A F, Martins R C, Quinta-ferreira R M (2013). Reuse of homogeneous Fenton’s sludge from detergent industry as Fenton’s catalyst. Journal of Advanced Oxidation Technologies, 16(2): 298–305
Sabhi S, Kiwi J (2001). Degradation of 2,4-dichlorophenol by immobilized iron catalysts. Water Research, 35(8): 1994–2002
Shahrifun N A, Ab’lah N N, Hussain H, Aris A, Omar Q, Ahmad N (2015). Reusability of Fenton sludge to reduce COD and color on palm oil mill secondary effluent (POMSE). Advanced Materials Research, 1113: 486–491
Shahrifun S A, Hussain H, Omar Q (2016). Optimization of solar Fenton Oxidation and comparison of recycle wet and dried Fenton sludge in treating palm oil mill secondary effluent. Jurnal Teknologi, 78(6–7): 61–67
Shen M, Huang Z, Luo X, Ma Y, Chen C, Chen X, Cui L (2020a). Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis. Chemical Engineering Journal, 396: 125238
Shen M, Huang Z, Qiu L, Chen Z, Xiao X, Mo X, Cui L (2020b). Recycling of Fenton sludge containing Ni as an efficient catalyst for tetracycline degradation through peroxymonosulfate activation. Journal of Cleaner Production, 268: 122174
Shukla P, Wang S, Sun H, Ang H, Tadé M (2010). Adsorption and heterogeneous advanced oxidation of phenolic contaminants using Fe loaded mesoporous SBA-15 and H2O2. Chemical Engineering Journal, 164(1): 255–260
Sillanpää M, Ncibi M C, Matilainen A (2018). Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. Journal of Environmental Management, 208: 56–76
Tan X, Liu Y, Gu Y, Xu Y, Zeng G, Hu X, Liu S, Wang X, Liu S, Li J (2016). Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresource Technology, 212: 318–333
Tang Y, Ren H, Yang P, Li H, Zhang J, Qu C, Chen G (2019). Treatment of fracturing fluid waste by Fenton reaction using transition metal complexes catalyzes oxidation of hydroxypropyl guar gum at high pH. Environmental Chemistry Letters, 17(1): 559–564
Tao X, Ma W, Li J, Huang Y, Zhao J, Yu J C (2003). Efficient degradation of organic pollutants mediated by immobilized iron tetrasulfophthalocyanine under visible light irradiation. Chemical Communications, 1(1): 80–81
Tong S, Shen J, Jiang X, Li J, Sun X, Xu Z, Chen D (2021). Recycle of Fenton sludge through one-step synthesis of aminated magnetic hydrochar for Pb2+ removal from wastewater. Journal of Hazardous Materials, 406: 124581
Umar M, Aziz H A, Yusoff M S (2010). Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Management, 30(11): 2113–2121
Voelker B M, Sulzberger B (1996). Effects of Fulvic acid on Fe (II) oxidation by hydrogen peroxide. Environmental Science & Technology, 30(4): 1106–1114
Walling C (1975). Fenton’s reagent revisited. Accounts of Chemical Research, 8(4): 125–131
Wang H, Xiao K, Yang J, Yu Z, Yu W, Xu Q, Wu Q, Liang S, Hu J, Hou H, Liu B (2020). Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron-rich sludge: A potential phosphorus fertilizer. Water Research, 174: 115629
Wang J, Wang S (2018). Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chemical Engineering Journal, 334: 1502–1517
Wang M, Zhao Z, Zhang Y (2018). Sustainable strategy for enhancing anaerobic digestion of waste activated sludge: driving dissimilatory iron reduction with Fenton sludge. ACS Sustainable Chemistry & Engineering, 6(2): 2220–2230
Wang M, Zhao Z, Zhang Y (2019). Disposal of Fenton sludge with anaerobic digestion and the roles of humic acids involved in Fenton sludge. Water Research, 163: 114900
Wang N, Zheng T, Zhang G, Wang P (2016). A review on Fenton-like processes for organic wastewater treatment. Journal of Environmental Chemical Engineering, 4(1): 762–787
Xiao S, Cheng M, Zhong H, Liu Z, Liu Y, Yang X, Liang Q (2020). Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chemical Engineering Journal, 384: 123265
Xu Z X, Song H, Deng X Q, Zhang Y Y, Xue-Qin M, Tong S Q, He Z X, Wang Q, Shao Y W, Hu X (2019). Dewatering of sewage sludge via thermal hydrolysis with ammonia-treated Fenton iron sludge as skeleton material. Journal of Hazardous Materials, 379: 120810
Yi Y, Huang Z, Lu B, Xian J, Tsang E P, Cheng W, Fang J, Fang Z (2020). Magnetic biochar for environmental remediation: A review. Bioresource Technology, 298: 122468
Yoo H, Cho S, Ko S (2001). Modification of coagulation and Fenton oxidation processes for cost-effective leachate treatment. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 36(1): 39–48
Yoon K, Cho D W, Tsang D C W, Bolan N, Rinklebe J, Song H (2017). Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresource Technology, 246: 69–75
Zhang H, Liu J, Ou C, Faheem, Shen J, Yu H, Jiao Z, Han W, Sun X, Li J, Wang L (2017). Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. Journal of Environmental Sciences-China, 53: 1–8
Zhang H, Xue G, Chen H, Li X (2018). Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere, 191: 64–71
Zhang H, Xue G, Chen H, Li X (2019a). Hydrothermal synthesizing sludge-based magnetite catalyst from ferric sludge and biosolids: Formation mechanism and catalytic performance. Science of the Total Environment, 697: 133986
Zhang J (2013). Fenton iron mud preparation of ferrous sulfate and polymeric ferric sulfate and its application. Dissertation for the Master Degree. Nanning: Guangxi University (in Chinese)
Zhang Y, Guo S, Zhou J, Li C, Wang G (2010). Flue gas desulfurization by FeSO4 solutions and coagulation performance of the polymeric ferric sulfate by-product. Chemical Engineering and Processing, 49(8): 859–865
Zhang M, Dong H, Zhao L, Wang D, Meng D (2019b). A review on Fenton process for organic wastewater treatment based on optimization perspective. Science of the Total Environment, 670: 110–121
Zhou R, Zhang W (2017). Reuse of ferric sludge by ferrous sulfide in the fenton process for nonylphenol ethoxylates wastewater treatment. Computational Water, Energy, and Environmental Engineering, 6(01): 89–96
Zhou Y, Fang X, Wang T, Hu Y, Lu J (2017). Chelating agents enhanced CaO2 oxidation of bisphenol A catalyzed by Fe3+ and reuse of ferric sludge as a source of catalyst. Chemical Engineering Journal, 313: 638–645
Zhu L, Shen D, Luo K H (2020). A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. Journal of Hazardous Materials, 389: 122102
Zhu S, Huang X, Ma F, Wang L, Duan X, Wang S (2018). Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environmental Science & Technology, 52(15): 8649–8658
Acknowledgements
We thank LetPub for its linguistic assistance during the preparation of this manuscript. L. Gao acknowledges the financial support of the National Natural Science Foundation of China (Grant No. 5210040121), Jiangsu Provincial Natural Science Foundation of Jiangsu Province (No. BK20210498) and the fellowship of China Postdoctoral Science Foundation (No. 2021M693420).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
• The sustainable approaches related to Fenton sludge reuse systems are summarized.
• Degradation mechanism of Fenton sludge heterogeneous catalyst is deeply discussed.
• The efficient utilization directions of Fenton sludge are proposed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Gao, L., Cao, Y., Wang, L. et al. A review on sustainable reuse applications of Fenton sludge during wastewater treatment. Front. Environ. Sci. Eng. 16, 77 (2022). https://doi.org/10.1007/s11783-021-1511-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-021-1511-6