Abstract
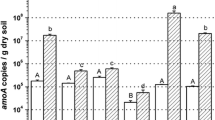
As low oxygen and high ultraviolet (UV) exposure might significantly affect the microbial existence in plateau, it could lead to a specialized microbial community. To determine the abundance and distribution of ammonia-oxidizing archaea (AOA) in agricultural soil of plateau, seven soil samples were collected respectively from farmlands in Tibet and Yunnan cultivating the wheat, highland-barley, and colza, which are located at altitudes of 3200–3800 m above sea level. Quantitative PCR (q-PCR) and clone library targeting on amoA gene were used to quantify the abundances of AOA and ammonia-oxidizing bacteria (AOB), and characterize the community structures of AOA in the samples. The number of AOA cells (9.34 × 107−2.32 × 108 g−1 soil) was 3.86–21.84 times greater than that of AOB cells (6.91 × 106−1.24 × 108 g−1 soil) in most of the samples, except a soil sample cultivating highland-barley with an AOA/AOB ratio of 0.90. Based Kendall’s correlation coefficient, no remarkable correlation between AOA abundance and the environmental factor was observed. Additionally, the diversities of AOA community were affected by total nitrogen and organic matter concentration in soils, suggesting that AOA was probably sensitive to several environmental factors, and could adjust its community structure to adapt to the environmental variation while maintaining its abundance.
Similar content being viewed by others
References
Wagner M, Rath G, Amann R, Koops H P, Schleifer K H. In-situ identification of ammonia-oxidizing bacteria. Systematic and Applied Microbiology, 1995, 18(2):251–264
Bock E, Wagner M. Oxidation of inorganic nitrogen compounds as an energy source. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K H, Stackebrandt E, editors. The Prokaryotes-An Evolving Electronic Resource for the Microbiological Community. 3rd ed. New York: Springer, 2001, 457–495
Hallam S J, Mincer T J, Schleper C, Preston C M, Roberts K, Richardson P M, DeLong E F. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biology, 2006, 4(4):520–536
Könneke M, Bernhard A E, de la Torre J R, Walker C B, Waterbury J B, Stahl D A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature, 2005, 437(7058):543–546
Hatzenpichler R, Lebedeva E V, Spieck E, Stoecker K, Richter A, Daims H, Wagner M. A moderately thermophilic ammoniaoxidizing crenarchaeote from a hot spring. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(6):2134–2139
Park B J, Park S J, Yoon D N, Schouten S, Sinninghe Damsté J S, Rhee S K. Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in coculture with sulfur-oxidizing bacteria. Applied and Environmental Microbiology, 2010, 76(22):7575–7587
Matsutani N, Nakagawa T, Nakamura K, Takahashi R, Yoshihara K, Tokuyama T. Enrichment of a novel marine ammonia-oxidizing archaeon obtained from sand of an eelgrass zone. Microbes Environ, 2011, 26(1):23–29
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(20):8420–8425
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol G W, Prosser J I, Schuster S C, Schleper C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature, 2006, 442(7104):806–809
Mosier A C, Francis C A. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environmental Microbiology, 2008, 10(11):3002–3016
Agogué H, Brink M, Dinasquet J, Herndl G J. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature, 2008, 456(7223):788–791
Kasuga I, Nakagaki H, Kurisu F, Furumai H. Predominance of ammonia-oxidizing archaea on granular activated carbon used in a full-scale advanced drinking water treatment plant. Water Research, 2010, 44(17 17SI):5039–5049
Park H D, Wells G F, Bae H, Criddle C S, Francis C A. Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Applied and Environmental Microbiology, 2006, 72(8):5643–5647
Limpiyakorn T, Sonthiphand P, Rongsayamanont C, Polprasert C. Abundance of amoA genes of ammonia-oxidizing archaea and bacteria in activated sludge of full-scale wastewater treatment plants. Bioresource Technology, 2011, 102(4):3694–3701
Wuchter C, Abbas B, Coolen M J, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl G J, Middelburg J J, Schouten S, Sinninghe Damsté J S. Archaeal nitrification in the ocean. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(33):12317–12322
Mussmann M, Brito I, Pitcher A, Sinninghe Damsté J S, Hatzenpichler R, Richter A, Nielsen J L, Nielsen P H, Müller A, Daims H, Wagner M, Head I M. Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(40):16771–16776
Nicol G W, Leininger S, Schleper C, Prosser J I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environmental Microbiology, 2008, 10(11):2966–2978
Zhang L M, Wang M, Prosser J I, Zheng Y M, He J Z. Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiology Ecology, 2009, 70(2):208–217
Di H J, Cameron K C, Shen J P, Winefield C S, O’Callaghan M, Bowatte S, He J Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience, 2009, 2(9):621–624
Verhamme D T, Prosser J I, Nicol G W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. The ISME journal, 2011, 5(6):1067–1071
Schauss K, Focks A, Leininger S, Kotzerke A, Heuer H, Thiele-Bruhn S, Sharma S, Wilke B M, Matthies M, Smalla K, Munch J C, Amelung W, Kaupenjohann M, Schloter M, Schleper C. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environmental Microbiology, 2009, 11(2):446–456
Zhao Z Q, Li S C, Gao J B, Wang Y L. Identifying spatial patterns and dynamics of climate change using recurrence quantification analysis: a case study of qinghai-tibet plateau. International Journal of Bifurcation and Chaos in Applied Sciences and Engineering, 2011, 21(04):1127–1139
Xu X K, Chen H, Levy J K. Spatiotemporal vegetation cover variations in the Qinghai-Tibet Plateau under global climate change. Chinese Science Bulletin, 2008, 53(6):915–922
Xu Z H, Chen C R, He J Z, Liu J X. Trends and challenges in soil research 2009: linking global climate change to local long-term forest productivity. Journal of Soils and Sediments, 2009, 9(2):83–88
Francis C A, Roberts K J, Beman J M, Santoro A E, Oakley B B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(41):14683–14688
Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Applied and Environmental Microbiology, 1997, 63(12):4704–4712
He J Z, Shen J P, Zhang L M, Zhu Y G, Zheng Y M, Xu M G, Di H J. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environmental Microbiology, 2007, 9(9):2364–2374
Gubry-Rangin C, Nicol G W, Prosser J I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiology Ecology, 2010, 74(3):566–574
Mertens B, Boon N, Verstraete W. Stereospecific effect of hexachlorocyclohexane on activity and structure of soil methanotrophic communities. Environmental Microbiology, 2005, 7(5):660–669
Wittebolle L, Vervaeren H, Verstraete W, Boon N. Quantifying community dynamics of nitrifiers in functionally stable reactors. Applied and Environmental Microbiology, 2008, 74(1):286–293
Juretschko S, Timmermann G, Schmid M, Schleifer K H, Pommerening-Röser A, Koops H P, Wagner M. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Applied and Environmental Microbiology, 1998, 64(8):3042–3051
Adair K L, Schwartz E. Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microbial Ecology, 2008, 56(3):420–426
Stein L Y, Arp D J. Loss of ammonia monooxygenase activity in nitrosomonas europaea upon exposure to nitrite. Applied and Environmental Microbiology, 1998, 64(10):4098–4102
Wang X, Wen X, Criddle C, Yan H, Zhang Y, Ding K. Bacterial community dynamics in two full-scale wastewater treatment systems with functional stability. Journal of Applied Microbiology, 2010, 109(4):1218–1226
Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Applied and Environmental Microbiology, 1996, 62(2):625–630
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, K., Wen, X., Chen, L. et al. Abundance and distribution of ammonia-oxidizing archaea in Tibetan and Yunnan plateau agricultural soils of China. Front. Environ. Sci. Eng. 8, 693–702 (2014). https://doi.org/10.1007/s11783-014-0635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0635-3