Abstract
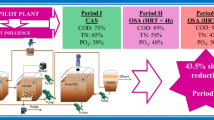
The oxic-settling-anaerobic (OSA) process is a promising wastewater treatment technique for efficiently reducing sludge production and improving the stability of process operation. In this paper, the possible factors of sludge reduction such as sludge decay, uncoupled metabolism, and anaerobic oxidation with low sludge production were discussed in the OSA process. It has been confirmed that sludge decay is the decisive cause in the OSA process, accounting for 66.7% of sludge production reduction. Sludge decay includes hydrolysis and acidogenesis of dead microorganisms and particle organic carbon adsorbed in sludge floc and endogenous metabolism. By batch experiments, it has been proven that there is energetic uncoupling in the OSA system since microorganisms were exposed to alternative anaerobic and aerobic environment. It accounts for about 7.5% of sludge production reduction. Soluble chemical oxygen demand (SCOD) released from the anaerobic sludge tank in the OSA process was used as the substrate for cryptic growth. The substrate was used for anoxic denitrifying, anaerobic phosphorus release, sulfate reduction, and methane production. These anaerobic reactions in the sludge anaerobic tank have lower sludge production than in the aerobic oxidation when equivalent SCOD is consumed, which may lead to approximately 23% of sludge reduction in the OSA process. It has been concluded that multiple causes resulted in the minimization of excess sludge in the OSA system. The microbial community structure and diversity of sludge samples from the CAS (conventional activated sludge) and OSA systems were investigated by 16 SrDNA PCR-DG-DGGE (polymerase chain reaction-double gradient-denaturing gradient gel electrophoresis). DGGE profile and cluster analysis showed more abundant species in the OSA system contrasting to microbial communities in the CAS system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Zhao Q L, Kugel G. Thermopholic/mesophilic digestion of sewage sludge and organic waste. J. Environ. Sci. Health., 1997, 31: 2211–2231
Liu Y, Tay J H. Strategy for minimization of excess sludge production from the activated sludge process. Biotechnol. Adv., 2001, 19: 97–107
Sabya S, Djafera M, Chen G H. Effect of low ORP in anoxic sludge zone on excess sludge production in oxicsettling-anoxic activated sludge process. Water Res., 2003, 37: 11–20
Chudoba P, Chdoba J, Capdeville B. The aspect of energetic uncoupling of microbial growth in the activated sludge process: OSA system. Water Sci. Technol., 1992, 26: 2477–2480
Chen G H, An K J, Saby S, et al. Possible cause of excess sludge reduction in an oxic-settling-anaerobic activated sludge process (OSA process). Water Res., 2003, 37: 3855–3866
American Public Health Association (APHA), American Water Works Association (AWWA). Standard Methods for the Examination of Water and Wastewater. 19th ed. Washington, USA: American Public Health Association, American Water Works Association, 1995
Frølund B, Griebe T, Nielsen P H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol., 1995, 43: 755–761
Dubois M, Gilles K A, Hamilton J K, et al. Colorimetric method for determination of sugars and related substances. Anal. Chem., 1956, 28: 350–356
Li X Z, Zhao Q L. Efficiency of biological treatment affected by high strength of ammonium-nitrogen in leachate and chemical precipitation of ammonium-nitrogen as pretreatment. Chemosphere, 2001, 41: 37–43
Zhou J Z, Bruns M A, Tiedje J M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol, 1996, 62(2): 316–322
Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16SrRNA. Appl. Environ. Microbiol, 1993, 59(3): 695–700
Chen T S, Li J J, Cen Y H, Sun G P. Analysis of microbial diversity and community composition after enrichment with deodorizing biofilter by DG-DGGE. Chi. J. Appl. Environ. Biol., 2006, 12(1): 113–117 (in Chinese)
Metacalf & Eddy, Inc. Wasterwater Engineering Treatment and Reuse. 4th ed. Beijing: Tsinghua University Press, 2003, 567–627
McSwain B S, Irvine R L, Hausner M, Wilderer P A. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microb., 2005, 71(2): 1051–1057
Herbert D, Elsworth R, Telling R C. The continuous culture of bacteria: A theoretical and experimental study. J. Gen. Appl. Microbiol., 1956, 114: 601
Wang W H, Jung Y J, Kiso Y, Yamada T, Min K S. Excess sludge reduction performance of an aerobic SBR process equipped with a submerged mesh filter unit. Process Biochem., 2006, 41: 745–751
Low E W, Chase H A, Milner M G, Curtis T P. Uncoupling of metabolism to reduce biomass production in the activated sludge process. Water Res., 2000, 34(12): 3204–3212
Rittmann B E, McCarty P L. Environmental Biotechnology Principles and Applications. Beijing: Tsinghua University Press, 2004, 165–493 (in Chinese)
Zehnder A J B, Wuhrmann K. Titatium(III) citrate as a nontoxic oxidation reduction buffering system for the culture of anaerobes. Science, 1976, 194: 1165–1166
Higgins M J, Novak J T. The effect of cations on the settling and dewatering of activated sludge. Water Environ. Res., 1997, 69(2): 215–224
Wang J F, Jin W B, Zhao Q L, et al. Study on performance of treating wastewater and anti-impaction in oxic-settling-anaerobic (OSA) process for minimization of excess sludge. Environmental Science, 2007, 28(11): 83–88 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Zhao, Q., Jin, W. et al. Mechanism on minimization of excess sludge in oxic-settling-anaerobic (OSA) process. Front. Environ. Sci. Eng. China 2, 36–43 (2008). https://doi.org/10.1007/s11783-008-0001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-008-0001-4