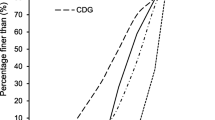
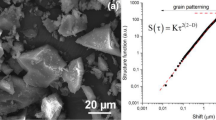
Abstract
Surface roughness of quartz particles was determined by measuring the specific surface area of particles. The wettability characteristics of particles were determined by measuring the flotation rate using a laboratory flotation cell. Experimental results show that the rod mill product has higher roughness than the ball mill product. For the particles with larger surface roughness, the flotation kinetics constant is also higher. Finally, empirical relationships between surface roughness (r) and the flotation kinetics constant (k) of quartz particles as k=A+Br+Cr 0.5lnr+D/lnr+E/r and k=A+Br are presented, in which A, B, C, D and E are constants related to experimental conditions and mineralogical properties of mineral.
Similar content being viewed by others
References
KUOPANPORTTI H, SUORSA T, DAHL O, NIINIMAKI J. A model of conditioning in the flotation of a mixture of pyrite and chalcopyrite ores [J]. International Journal of Mineral Processing, 2000, 59(4): 327–338.
KLIMPEL R R. Optimizing the industrial flotation performance of sulfide minerals having some natural floatability [J]. International Journal of Mineral Processing, 2000, 58 (1–4): 77–84.
XU M. Modified flotation rate constant and selectivity index [J]. Minerals Engineering, 1998, 11(3): 271–278.
OLIVEIRA J F, SARAIVA S M, PIMENTA J S, OLIVEIRA A P A. Kinetics of pyrochlore flotation from Araxa mineral deposits [J]. Minerals Engineering, 2001, 14(1): 99–105.
AGAR G E, CHIA J, REQUIS C L. Flotation rate measurements to optimize an operating circuit [J]. Minerals Engineering, 1998, 11(4): 347–360.
ÇILEK E C. Estimation of flotation kinetic parameters by considering interactions of the operating variables [J]. Minerals Engineering, 2004, 17(1): 81–85.
SZLEIFER I, SHAUL A B, GELBERT W M. Chain statistics in micelles and bilayers: Effects of surface roughness and internal energy [J]. J Chem Phys, 1986, 85(9): 5345–5358.
HOGG R. Characterization of mineral surfaces [M]//SOMASUNDARAN P Ed, Fine Particle Processing, Vol. I. New York: Society of Mining Engineers of AIME, 1980: 492–524.
JAYCOCK M J, PARFITT G D. Chemistry of interfaces [M]. Chichester, UK: Ellis Horwood Publications, 1981: 156–161.
HODSON M E, LEE M R, PARSONS I. Origins of the surface roughness of unweathered alkali feldspar grains [J]. Geochimica et Cosmochimica Acta, 1997, 61: 3885–3896.
HICYILMAZ C, ULUSOY U, BILGEN S, YEKELER M. Flotation responses to the morphological properties of particles measured with three-dimensional approach [J]. International Journal of Mineral Processing, 2005, 75: 229–236.
KELLY Z G, SPOTTISWOOD D J. Introduction to Mineral Processing [M]. New York: Wiley, 1982: 117.
ORUMWENSE O A, FORSSBERG E. Surface and structural changes in wet ground minerals [J]. Powder Technology, 1991, 68: 23–29.
OLIVER J F, HUH C, MASON S G. An experimental study of some effects of solid surface roughness on wetting [J]. Colloids and Surfaces, 1980, 1: 79–104.
DUCKER W A, PASHLEY R M, NINHAM B W. The flotation of quartz using a double-chained cationic surfactant [J]. Journal of Colloid and Interface Science, 1988, 128: 66–75.
FENG D, ALDRICH C. A comparison of the flotation of ore from the Merensky Reef after wet and dry grinding [J]. International Journal of Mineral Processing, 2000, 60: 115–129.
EXTRAND C W. Criteria for ultralyophobic surfaces [J]. Langmuir, 2004, 20: 5013–5018.
KRASOWSKA M, MALYSA K. Kinetics of bubble collision and attachment to hydrophobic solids: I. Effect of surface roughness [J]. International Journal of Mineral Processing, 2007, 81: 205–216.
VIEIRA A M, PERES A E C. The effect of amine type, pH, and size range in the flotation of quartz [J]. Minerals Engineering, 2007, 20: 1008–1013.
ANFRUNS J F, KITCHENER J A. Rate of capture of small particles in flotation [J]. Trans Inst Min Metall, 1977, 86: C9–C15.
AHMED M M, STECHEMESSER H, MABROUK S A, IBRAHIM G A, TARSHAN M M. The relationship between the surface roughness, shape and detachment force of particles from the liquid/gas interface using centrifuge method [C]// 49 Berg-und Huettenmaennischer Tag, Kolloquium 2, Partikeltechnologie, Freiberg, Germany, 1998: 207–223.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimi, M., Aslani, M.R. & Rezai, B. Influence of surface roughness on flotation kinetics of quartz. J. Cent. South Univ. Technol. 19, 1206–1211 (2012). https://doi.org/10.1007/s11771-012-1130-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1130-2