Abstract
Purpose
Distress screening has become mandatory and essential in comprehensive cancer care. We evaluated an electronic psycho-oncological adaptive screening (EPAS) which assesses objective indicators of care needs and subjectively perceived care needs and subsequently provides patient feedback with individualized recommendations about psychosocial care services.
Methods
Patients were assessed within clusters, i.e., different oncological facilities of the competence network of the University Cancer Center Hamburg (UCCH). Patients in the intervention arm underwent the screening, controls received standard care. Patients were assessed at baseline (t0), 3-month (t1), and 6-month (t2) follow-up. Outcomes included information level and use of/access to nine psychosocial services at UCCH, well-being (GAD-7, PHQ-9, SF-8), and treatment satisfaction (SCCC). Conditional linear and logistic regressions were used to identify screening effects at t1 and t2.
Results
Of 1320 eligible patients across 11 clusters, 660 were included (50%). The average age was 60 years; 46% were female. The intervention was associated with increased information level for all psychosocial services at t1 and t2 (all p < .001), increased use in some of these services at t1 and t2, respectively (p ≤ .02), and better evaluation of access (e.g., more recommendations for services provided by physicians, p < .01). At t2, the intervention was associated with a lower level of satisfaction with disease-related information (p = .02).
Conclusions
EPAS may improve information about psychosocial services as well as utilization of and access to these services. The effect on information level seems not to be generalizable to other aspects of oncological care. Future studies should incorporate novel technologies and condense the procedure to its core factors.
Implications for Cancer Survivors: The screening may help to enhance self-management competencies among cancer survivors.
Trial registration
The trial was retrospectively registered (2/2021) at ClinicalTrials.gov (number: NCT04749056).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
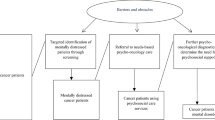
Due to multiple challenges in all areas of life, many cancer patients show elevated levels of mental burden such as depression or anxiety [1, 2]. Such symptomatology, conceptualized under the broader term distress [3], may worsen quality of life and even medical outcomes such as morbidity and mortality [4]. Nevertheless, many distressed patients are not recognized by the treating clinicians [5] and left untreated even though effective psychosocial interventions exist [6]. Therefore, screening for distress to detect those in need is considered mandatory in comprehensive cancer care [7]. In recent years, the general feasibility of electronic distress screenings in oncological routine care has been repeatedly demonstrated [8,9,10,11,12].
Previous findings on the effects of screenings on well-being, communication and referral are mixed, and thus the general benefit of screening is often argued [13]. Discrepant findings, however, may be caused by barriers that impede the usefulness of the screenings, such as lack of qualification of the physicians in interpreting results or a lack of transformation of screening results into individualized support plans [13,14,15]. Resolving such barriers requires extensive and repeated training [14, 16], which in turn may hamper the long-term effectiveness of a screening program. Therefore, reducing the burden for the medical staff to a minimum by facilitating referral to the psychosocial services within the respective institution may support long-term implementation of such a program.
Another important issue in screening is the time that patients need to complete the self-report questionnaires. Previous screenings mostly use conventional instruments based on classical test theory such as the Patient Health Questionnaire-9 (PHQ-9) [17] or the Generalized Anxiety Disorder-7 (GAD-7) [18] which present the same set of items to all participants. An alternative to these fixed-length questionnaires is the application of computerized-adaptive tests (CATs) based on item response theory that considers both item characteristics and the individual response pattern. CATs work in such way that they only present those items to individual respondents that are most relevant to them, thereby selecting an item subset from a larger pool of items (i.e., item bank). Such tailoring reduces respondent burden and thus may result in improved acceptability among patient populations and healthcare providers [19].
From a conceptual perspective, current distress screenings mostly rely on objective indicators of supportive care needs, i.e., they use patient-reported levels of distress to decide about further actions. This approach assumes that such objective indicators are closely linked to the subjectively perceived care need of a patient. However, recent research has revealed that objective indicators of care needs and subjectively perceived care needs are not necessarily related: For example, a study including a large population (n = 4020) demonstrated that only 51% of the patients with elevated distress levels reported a need for psychosocial support, whereas 26% of those without elevated distress levels reported such a need [20]. Therefore, both distress values as objective indicators and subjectively perceived care needs may be important to be included in distress screening programs.
We developed an electronic psycho-oncological adaptive screening program (EPAS) which incorporates both levels of distress and subjectively perceived care needs and subsequently provides immediate patient feedback with individualized recommendations about psychosocial care services at the care facility. We evaluated the screening by testing the effect of EPAS on all steps of the screening process, i.e., starting from information level about the psychosocial services up to mental health outcomes.
Methods
Study design and participants
Patients were assessed within clusters, i.e., different inpatient and outpatient cancer care facilities of the competence network of the University Cancer Center Hamburg (UCCH). Initially, we aimed to form matched pairs of clusters according to similar characteristics in order to allocate comparable clusters to the intervention and control condition, respectively. However, such a matching did not seem feasible due to differences in numbers and characteristics of patients across clusters. Therefore, we addressed the cluster bias by aiming to assess a similar number of patients in each cluster for each condition. To reduce the risk of any interfering effect between patients from different conditions, we prevented any overlap of conditions among patients that were in the same (waiting) room. This was accomplished by different measures, e.g., by suspending recruitment before changing the condition until the set of patients had completely changed or by assessing in spatially separated locations within the respective cluster.
Participants were eligible if they were (i) diagnosed with any malignancy according to ICD-10 irrespective of remission or treatment status or history of relapse, (ii) aged 18 years or older, (iii) able to read and speak German, and (iv) accessible for the research assistants (e.g., excluding those who were currently in isolation to prevent infection). For the intervention, patients needed to be skilled for using a tablet. Patients with any impairments interfering with the ability to give informed consent were excluded.
Patients were checked for eligibility via information provided by the treating physician and review of the medical chart. They were consecutively recruited by trained research assistants during their visit at the respective care facility for either treatment or medical check-up. To avoid repeated study invitation, the list of current patients in the respective cluster was compared with the list of previous participants before each recruitment. Each patient received only one condition; the intervention consisted of one screening.
The baseline assessment (t0) for the intervention group was completed using a tablet computer at the respective cancer care facility; during this session, both the measures of the intervention (EPAS) and the baseline study outcomes were assessed. The control group received a paper pencil questionnaire to be completed at the facility or at home using a pre-stamped envelope. At 3 months (t1) and 6 months (t2) follow-up, all participants were sent paper–pencil questionnaires by mail and reminded if the questionnaire were not returned within 2 weeks. The intervention was non-randomized and unblinded.
All participants provided written informed consent. The study protocol was approved by the ethics committee of the medical chamber of Hamburg (PV4371). The study was retrospectively registered (2/2021) at ClinicalTrials.gov (number: NCT04749056).
Intervention condition (EPAS)
Principle and procedure: EPAS (electronic psycho-oncological adaptive screening) is a tablet-based screening application consisting of (i) three adaptive tests assessing levels of distress (= objective indicators of care needs) and (ii) one supportive care checklist to explicitly report the need for specific psychosocial services (= subjectively perceived care needs). EPAS provides immediate feedback via a printed results page, which presents and interprets the level of distress and contains individualized recommendations for psychosocial services. The results pages were printed by research assistants immediately after the screening on a mobile printer and given to the participants together with a brochure containing information about all psychosocial services available at the UCCH. The treating physicians received a slightly different results page, but were not expected to discuss these with the patient unless they were highly distressed (see Algorithm section). Before and during the screening, patients were explained how to use the program by the research assistant and were supported if needed.
Measures within EPAS: To assess levels of distress, CATs were applied, i.e., the depression CAT (D-CAT) to assess depression [21], the anxiety CAT (A-CAT) to assess anxiety [22], and stress CAT (S-CAT) to assess stress which was further divided into two separate CATs on stress exposure and stress reaction, respectively [23]. From the respective item bank, only items with the highest information value were selected according to both item characteristics and individual response pattern. The presentation of items ended if the standard error was ≤ 3.2 or a maximum of 10 items was reached. Completion time for each of the CAT instruments ranged from 96 s (D-CAT) to 151 s (A-CAT), the mean completion time for all 4 adaptive screening instruments was 8.3 min. The mean number of items ranged between 6 (D-CAT) and 10 (S-CAT, stress exposure), with corresponding standard errors of the respective theta values being 3.0 and 4.1, respectively. In addition to the CAT instruments, patients filled in an internally developed checklist to report supportive care needs across different topics, e.g., psycho-oncological or social counseling (Table S1).
Structure and content of results page: The results page contained (i) the extent of distress, (ii) a summary of the reported supportive care needs, and (iii) individualized recommendations for the use of psychosocial services at the UCCH based on the care needs. For all adaptive tests, aids to interpret the respective levels were provided: For the D-CAT and the A-CAT, categories of “low,” “medium,” and “high” distress were defined. For the D-CAT, these categories had been derived from a standardization study aimed at defining a common metric for depression based on data from psychosomatic patients and the general population [24]. For the A-CAT, no norm data were available: Therefore, we selected patients from a psychosomatic sample that had completed the GAD-7 [18] alongside the A-CAT (unpublished data). In doing so, we were able to determine the respective theta values that corresponded to the GAD-7 scores of 5 (low), 10 (medium), and 15 (high). In contrast, no data were available as a gold standard for assigning severity categories to the S-CAT: To facilitate data interpretation, we instead used a patient sample diagnosed with either adjustment disorder or burn-out syndrome and applied the respective mean (separately for stress reaction and stress exposure) as a reference value (unpublished data). Of note, the patient and physician versions of the feedback page differed slightly: Patients were explicitly referred to the information brochure which they received during the screening. In contrast, the distress categories in the physician version were illustrated with colors (low = green; medium = yellow; high = red) and contained specific information for highly distressed patients (see Algorithm section).
Algorithm for results page: The supportive care needs reported in the checklist were transformed into concrete recommendations for using the adequate psychosocial service at the UCCH (e.g., a reported need for support in the topic “return to work” resulted in a recommendation to use the social service; Table S1). Highly distressed patients, i.e., those falling into the category “high” in the A-CAT or D-CAT, were recommended to use psycho-oncological service irrespective of whether they had reported such a need in the checklist. The treating physicians of highly distressed patients were recommended on their results page to talk with the patient about his/her psychosocial condition and were given further suggestions for such an appointment (e.g., check for medical reasons for distress, encourage patients to use psycho-oncological service).
Control condition
Controls completed all three assessments (i.e., at t0, t1, and t2) via paper pencil questionnaires. From the measures used in the EPAS intervention (CATs and supportive care checklist), the controls completed a paper pencil version of the supportive care checklist, but did not complete CATs which require an electronic assessment. Neither patients nor physicians received any feedback of the results. Psychosocial services were recommended by the physicians on their own discretion only. Patients did not receive the information brochure. Nevertheless, patients had the same access to all psychosocial services as the intervention group, and the information brochure was visible and available at all centers of the UCCH.
Outcomes
Since this screening approach had not been tested before, we were equally interested in effects of the screening across all levels of psychosocial care, i.e., from being informed about psychosocial services up to their potential benefits for mental health. Therefore, we decided against hierarchization in primary and secondary outcomes. Nevertheless, we note that the outcome information level was considered the basic requirement for all other outcomes and thus may represent the central outcome.
Information level about psychosocial services
We internally developed single item scales assessing the level of information for each of the psychosocial services which were available at the UCCH. On a five-point Likert scale ranging from 0 (“I do not even know that such a program exists”) to 4 (“very well”), patients rated how well they feel informed about the respective service.
Use of psychosocial services/evaluation of access
Analogously to the single item scales to assess information level, we internally developed binary items to assess the respective use of the psychosocial services available at the UCCH (yes/no).
To evaluate the access to the services, we internally developed binary items (yes/no) to assess whether any (i) communication with the physicians about supportive/complementary care needs, (ii) recommendations by the physicians for specific psychosocial services, (iii) concrete offers by the physicians to use a psychosocial service, or (iv) request by the physicians for a consultation service had taken place.
Well-being and treatment satisfaction
All of these outcomes were assessed in both the intervention and control group using validated questionnaires. In detail, well-being was assessed via depression (PHQ-9 [17]), anxiety (GAD-7 [18]), and quality of life (SF-8 [25]), while treatment satisfaction was assessed via the Satisfaction with Comprehensive Cancer Care (SCCC) questionnaire [26].
Sociodemographic and medical data
Sociodemographic and medical data were obtained from self-report and medical chart, respectively.
Administration of outcomes
Since more than half of the patients were diagnosed very recently (≤ 3 months), care relevant outcomes, i.e., items related to the psychosocial services at the UCCH and treatment satisfaction, were not expected to be applicable at t0 and thus were assessed at t1 and t2 only. Variables on the evaluation of access to the services were assessed once (t1). Variables on well-being (depression, anxiety, and quality of life) were assessed at all measurement points. Given that the other outcomes were not assessed at t0, we did not include baseline scores of the well-being variables in the respective analyses in order to use the same covariates for each analysis and thus to ensure comparability of the findings.
Power analysis
The study aimed to test for group differences between conditions, separately for t1 and t2. To detect an expected small to medium group difference in level of information (effect size = 0.3) with a power of 80%, sample sizes of 176 patients in each group were needed. We initially expected a drop-out rate of 30% until t2, resulting in a minimum of n = 251 per group (ntotal = 502).
Statistical analyses
We provided descriptive statistics for sociodemographic and medical data. To investigate baseline differences between conditions, comparisons were conducted via logistic regression and t tests for binary and continuous data, respectively. The same tests were applied to investigate representativeness of the sample at the follow-up time points by comparing drop-outs vs. study completers.
To investigate the group effect of the intervention, we applied linear (information level/well-being/treatment satisfaction) and logistic (use of service/evaluation of access) regression analyses, separately for t1 and t2.
All models were controlled by covariates. Given that group effects were investigated for both t1 and t2, an empirical approach to select covariates according to differences between conditions or between drop-outs and completers would have resulted in different covariates at both measurement points and thus would have limited comparability of the findings. Therefore, covariates were selected based on theory. In detail, we selected the two variables which were supposed to be particularly important for care relevant outcomes [20] and were also used as the two domains within the EPAS, i.e., objective indicators of care need (= level of general distress measured with the distress thermometer (DT) [27] at t0) and subjectively perceived care need (= percentage of reported needs on the supportive care checklist at t0). Additionally, we included gender and age as central sociodemographic variables. Covariates were checked for multicollinearity (bivariate correlations r < 0.7).
For the linear regression models, we also provide ΔR2 to indicate the change in explained variance of the model after having added the intervention variable (calculation: R2whole model—R2model without intervention variable). For the logistic regressions, we report odds ratios (OR): values > ( <) 1 mean that the odds to experience the respective event in the intervention group are higher (lower) than in the control group. For example, an OR = 4 means that for every 4 persons that experience the event in the intervention group, 1 person will experience the event in the control group [28]. The alpha level was set at 0.05. Listwise deletion was applied; analyses were conducted using SPSS (Version 25).
Deviations from initial study proposal
Given the retrospective trial registration, we attached a translation of the synopsis of the initial study proposal to ensure maximum transparency (Table S2). Any points by which the final report of the study deviates from the initial study proposal are listed and explained in Table S3.
Results
Participant flow
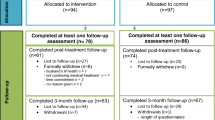
We recruited from December 2013 to December 2014; data collection was completed in July 2015. Eleven clusters participated in the study, among which 1784 patients were checked for eligibility (Fig. 1). In the intervention arm, 333 out of 673 eligible patients were included (49%). In the control arm, 327 out of eligible 647 patients were included (51%). The drop-out rate until t2 was 69% and 53% for the intervention and the control condition, respectively. For additional information on included clusters, see Table S4.
Flow chart. UCCH, University Cancer Center Hamburg. aClusters were included if they (i) were primary oncology facilities, (ii) treating a high number of patients, and (iii) agreed to participate in the study; each cluster received each condition except for the cluster “Marienkrankenhaus—Private station” which only received control condition owing to too few participants. bSevere physical/mental/cognitive impairment (n = 193), isolated (n = 18), insufficient language skills (n = 68), incompetence to use tablet as assessed by physicians (n = 9). cPhysical/mental burden (n = 32), organizational issues (n = 19), no interest (n = 171), other reasons (n = 59). dSevere physical/mental/cognitive impairment (n = 130), isolated (n = 21), insufficient language skills (n = 23), underage (n = 2). ePhysical/mental burden (n = 14), organizational issues (n = 8), no interest (n = 66), other reasons (n = 5). fWithin clearance of the final data, any cases with unrestorable documentation errors or missing/unclear information on either age, gender, or diagnosis were deleted. gPatient loss mostly due to not sending back the questionnaires or having deceased
Compared to study completers, drop outs were more likely to be in the intervention group (p < 0.001) and to have metastases (p = 0.03), palliative treatment (p < 0.001), and a history of relapse (p = 0.01). They were less likely to have a partner (p = 0.03) or a hematological cancer diagnosis (p = 0.001) and had a longer time since diagnosis (p = 0.04).
Baseline sample characteristics
The average age was 60 years, 46% were female (Table 1). The most frequent cancer types were hematological and colorectal (32 and 12%, respectively), and more than half or participants were diagnosed within the last 3 months.
Compared to the controls, patients in the intervention group were younger, better educated, less distressed, more likely to be inpatient and to be treated with surgery and radiation, but less likely to be in a relationship (Table 1).
Effect on level of information
At both t1 and t2, the intervention was significantly associated with a higher level of information across all nine psychosocial services (Table 2). ΔR2 indicated that the intervention variable increased the explained variance of the whole model with up to 23% (cancer survivorship program at t1).
Effect on use of psychosocial services and the evaluation of access
At t1 and t2, the intervention was significantly associated with more frequent use of the activity and sports programs (Table 3). Furthermore, the intervention was significantly associated with more frequent use of the service complementary medical lesson at t2. The OR of the significant effects reached up to 2.2 and 4.1 at t1 and t2, respectively.
The intervention was associated with better evaluation regarding the access of the services. In detail, patients more frequently talked with their physician about supportive care services, received more recommendations on such services, and were more often given concrete offers to use these psychosocial services. The OR of the significant effects ranged from 2.0 to 2.4.
Effect on well-being and patient satisfaction
The intervention variable was not significantly associated with any measures of well-being (Table 4). Patients in the intervention group were significantly less satisfied with their level regarding disease-related information; however, the intervention variable only added 2% of explained variance to this overall model effect.
Discussion
Main findings
This cluster intervention showed that an electronic psycho-oncological screening with immediate patient feedback was associated with an increased level of information about different psychosocial services, better support by physicians to access these services, and increased use in one and two of these services after 3 and 6 months, respectively.
Integration into previous research
Comparability with previous studies is limited: Previous studies on online distress screenings mostly tested feasibility, but did not assess the effect on specific outcomes, e.g., [8,9,10,11,12, 29]. Furthermore, we identified self-management programs with tailored patient feedback among recent reviews (n = 12 [30] and n = 13 [31]), but these programs primarily addressed medical management and/or consisted of several assessments. A recent RCT (n = 625 survivors) on an online screening with subsequent individualized feedback on supportive care options was conceptually similar to ours [32]. Nevertheless, they used different outcomes (primary outcome: patient activation) and thus is hard to compare with our findings.
We found large effects of the screening on information level across all types of psychosocial services. Since information level forms the basis to empower patients in using support, this result is central for our initial aim to improve health-related self-management. Several aspects within the screening may have contributed to this effect, including the communication with the study assistants or the provision of the information brochure. For example, additional analyses (data not shown) revealed a strong positive intervention effect on the degree to which the brochure had been read. In addition, more than half of the patients in the control condition reported that they did not even know that such an information brochure existed even though brochures were available throughout the facilities. Such findings indicate that future research is needed to condense the screening procedure to its core elements, e.g., determine the extent by which the information level could be achieved by handing out the brochure alone.
The screening was associated with a more frequent use in some of the psychosocial services. The effect may be partly explained by the improved support by physicians in accessing these services in terms of more frequent communication, recommendation, and referral to consultation-liaison services. The higher referral rate which resulted from the screening is in line with a recent screening intervention using a stepped-care approach [16]. The OR reached up to 4 (service complementary medical lesson after 6 months), meaning that for every 4 people that used this specific service in the intervention group, only one person used this service in the control group. For most services, however, we did not find an effect: As an explanation, the benefit may depend on patient characteristics such as self-efficacy or health literacy [33] and thus respective effects may only be found for certain subgroups of patients. As a second explanation, it is to note that patients were relatively shortly after their diagnosis: It is possible that patients need time to adjust to the new situation, to recognize their need for supportive care, and finally to decide for any professional support. Since OR may be overestimated in small sample sizes [34], we note that the comparability of effects between time points is limited given different sample sizes.
In line with previous research, we did not find any screening effect on well-being [16, 35,36,37,38]. One explanation may be a floor effect. For example, one of the few studies (n = 116 breast cancer patients) which found a significant improvement in anxiety and depression pre-selected patients with high distress (DT ≥ 7) [39], which was only true for 31% of the patients in our study (data not shown). Furthermore, the study design might not have been adequate to detect effects on well-being: Given that we could not control whether and which psychosocial service was actually used and that the control group had the same access to the services, an equal intervention effect related to these outcomes could not be investigated.
The screening was associated with a lower level of satisfaction with disease-related information, which may first seem contra-intuitive. Two explanations, however, seem reasonable: First, this 7-item scale assessed mainly medical information, with only one item assessing the information level related to psychosocial services; therefore, our screening was not adequate to improve this specific outcome. Second, patients in the intervention group may have used their elevated level of information regarding psychosocial services as a reference for “satisfying information” and thus may have been “stricter” in their evaluation of the information level with medical aspects.
Implications
In light of the lack of a direct effect on well-being, some authors argue if an implementation of such a program is warranted [40]. However, we think that such a decision needs to consider both efforts and benefits: In the current approach, we kept the effort of the medical staff as low as possible. This in turn may help to reduce institutional implementation barriers such as resources, training/education, or organizational readiness [41, 42]. Furthermore, we found robust effects in strengthening patient autonomy through information and improving awareness in physicians. Therefore, the benefits may speak in favor for the implementation of such a program.
Several adaptations may further optimize the screening. First, the project started in 2013 and thus the program needs to be adapted to the new technological developments and made applicable on mobile devices such as smartphones. In doing so, a specific app may guide the patient through the program, and the results might be directly sent to the patients and physicians which would further improve efficiency. Detailed assessment of the number and extent of help provided by the research team in using such apps will help to improve usability. For institutions with fewer psychosocial services, the algorithm needs to be adapted, e.g., by referring patients to online support services, websites of ambulatory psycho-oncologists, or specific self-help groups. Given that the screening procedure consisted of several aspects, it remains unclear which elements are central for the respective benefits of the screening. Future studies need to identify such core elements to improve its efficiency. Secondary analyses may investigate moderator effects such as time since diagnosis or remission status to identify subgroups for which the screening is particularly effective.
Strengths
We provided an innovative approach by combining several aspects such as (i) focus on psycho-oncological outcomes, (ii) use of adaptive screening instruments, (iii) assessing both levels of distress and subjectively perceived care needs, and (iv) making use of already existing psychosocial services at the healthcare facility. In doing so, we tried to optimize efficiency for all stakeholders which may increase the likelihood of the screening to be persistently applied in routine care settings. Given these strengths, our study including eleven clusters in different clinics and cancer care facilities may provide a novel approach for health services research in oncology.
Limitations
We had a moderate response rate. As an explanation, patients had a mean age of 60, and thus a part of the eligible patients may not have been used to deal with digital devices back in 2013. It is also to note that patients were approached during their visit in the facility for treatment or medical check-up and thus they may not have been responsive for study participation. Among responders, the drop-out rate was relatively high, which may be caused by two issues: First, the majority of the sample was in a severe medical condition, with more than half being in palliative treatment. In fact, dropouts were more likely to have a worse physical status at baseline (i.e., more often metastases and relapse), which implies that many patients did not continue with the study because they felt too burdened to complete the assessments. Second, the questionnaires were relatively long and may have reduced motivation to complete the study: This assumption is supported by the fact that we observed a higher number of drop-outs in the intervention condition, for which the baseline assessment including the screening was longer than in the control group. Given that both conditions were applied in the clusters, we cannot rule out a carry-over effect among treating physicians in that they had become more aware of psychosocial issues in treating the control group after having started in the intervention condition. Even though we recruited more than patients than originally planned, we did not reach the minimum sample size in the final analyses, which may have limited test power. We also note that the outcomes on the psychosocial services were internally developed and thus might have lacked sensitivity. Finally, the trial was retrospectively registered. To ensure maximum transparency, however, we attached a translated version of the synopsis of the initial study proposal and provided details and reasons for any deviations.
Conclusions
This study demonstrated that an electronic adaptive screening program for cancer survivors may enhance patient autonomy by increasing their information level and the use of psychosocial services and may improve the support by physicians to access these services. Future studies are needed to explore its use on mobile devices and to reduce the procedure to its core factors.
Code availability
The data and the code that support the findings of this study are available upon request from the authors.
Change history
04 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11764-021-01148-x
References
Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46–53.
Kuba K, Esser P, Mehnert A, et al. Risk for depression and anxiety in long-term survivors of hematologic cancer. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2019;38:187–95. https://doi.org/10.1037/hea0000713.
Bultz BD, Carlson LE. Emotional distress: the sixth vital sign–future directions in cancer care. Psychooncology. 2006;15:93–5. https://doi.org/10.1002/pon.1022.
Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–810. https://doi.org/10.1017/S0033291709992285.
Fallowfield, L, et al. Psychiatric morbidity and its recognition by doctors in patients with cancer.
Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013;31:782–93.
Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:1160–77. https://doi.org/10.1200/JCO.2011.39.5509.
Li M, Macedo A, Crawford S, et al. Easier said than done: keys to successful implementation of the distress assessment and response tool (DART) program. Journal of oncology practice. 2016;12:e513–26. https://doi.org/10.1200/JOP.2015.010066.
Schepers SA, Sint Nicolaas SM, Maurice-Stam H, et al. First experience with electronic feedback of the psychosocial assessment tool in pediatric cancer care. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25:3113–21. https://doi.org/10.1007/s00520-017-3719-3.
Smith SK, Rowe K, Abernethy AP. Use of an electronic patient-reported outcome measurement system to improve distress management in oncology. Palliat Support Care. 2014;12:69–73. https://doi.org/10.1017/S1478951513000345.
Schäffeler N, Pfeiffer K, Grischke EM, et al. Akzeptanz und Reliabilität eines elektronischen psychoonkologischen Screenings bei Patientinnen mit Brustkrebs: eine randomisiert-kontrollierte Studie. Psychother Psychosom Med Psychol. 2013;63:374–80. https://doi.org/10.1055/s-0032-1333301.
Zebrack B, Kayser K, Sundstrom L, et al. Psychosocial distress screening implementation in cancer care: an analysis of adherence, responsiveness, and acceptability. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1165–70. https://doi.org/10.1200/JCO.2014.57.4020.
Mitchell AJ. Screening for cancer-related distress: when is implementation successful and when is it unsuccessful? Acta Oncol. 2013;52:216–24.
Carlson LE. Screening alone is not enough: the importance of appropriate triage, referral, and evidence-based treatment of distress and common problems. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3616–7. https://doi.org/10.1200/JCO.2013.51.4315.
McCarter K, Britton B, Baker AL, et al. Interventions to improve screening and appropriate referral of patients with cancer for psychosocial distress: systematic review. BMJ open 2018 8.
Singer S, Danker H, Roick J, et al. Effects of stepped psychooncological care on referral to psychosocial services and emotional well-being in cancer patients: a cluster-randomized phase III trial. Psychooncology. 2017;26:1675–83. https://doi.org/10.1002/pon.4492.
Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71–7.
Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Medical care 2008:266–74.
Fischer HF, Klug C, Roeper K, et al. Screening for mental disorders in heart failure patients using computer-adaptive tests. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23:1609–18. https://doi.org/10.1007/s11136-013-0599-y.
Faller H, Weis J, Koch U, et al. Perceived need for psychosocial support depending on emotional distress and mental comorbidity in men and women with cancer. J Psychosom Res. 2016;81:24–30.
Fliege H, Becker J, Walter OB, Rose M, Bjorner JB, Klapp BF. Evaluation of a computer-adaptive test for the assessment of depression (D-CAT) in clinical application. Int J Methods Psychiatr Res. 2009;18:23–36.
Becker J, Fliege H, Kocalevent R-D, et al. Functioning and validity of A computerized adaptive test to measure anxiety (A-CAT). Depress Anxiety. 2008;25:E182–94.
Kocalevent R-D, Rose M, Becker J, et al. An evaluation of patient-reported outcomes found computerized adaptive testing was efficient in assessing stress perception. J Clin Epidemiol. 2009;62:278–87.
Wahl I, Löwe B, Bjorner JB, et al. Standardization of depression measurement: a common metric was developed for 11 self-report depression measures. J Clin Epidemiol. 2014;67:73–86. https://doi.org/10.1016/j.jclinepi.2013.04.019.
Ellert U, Lampert T, Ravens-Sieberer U. Messung der gesundheitsbezogenen Lebensqualität mit dem SF-8. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2005 48:1330-7.
Esser P, Sautier L, Sarkar S, et al. Development and preliminary psychometric investigation of the German satisfaction with comprehensive cancer care (SCCC) questionnaire. Health Qual Life Outcomes. 2021;19:147. https://doi.org/10.1186/s12955-021-01784-y.
Mehnert A, Müller D, Lehmann C, Koch U. Die deutsche version des NCCN distress-thermometers: empirische Prüfung eines screening-instruments zur erfassung psychosozialer belastung bei krebspatienten. Z Psychiatr Psychol Psychother. 2006;54:213–23.
Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76:857–61.
Dinkel A, Berg P, Pirker C, et al. Routine psychosocial distress screening in radiotherapy: implementation and evaluation of a computerised procedure. Br J Cancer. 2010;103:1489–95. https://doi.org/10.1038/sj.bjc.6605930.
Warrington L, Absolom K, Conner M, et al. Electronic systems for patients to report and manage side effects of cancer treatment: systematic review. J Med Internet Res. 2019;21:e10875. https://doi.org/10.2196/10875.
Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2018;26:337–51. https://doi.org/10.1007/s00520-017-3882-6.
van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol. 2020;21:80–94. https://doi.org/10.1016/S1470-2045(19)30675-8.
van der Hout A, Holtmaat K, Jansen F, et al. The eHealth self-management application ‘Oncokompas’ that supports cancer survivors to improve health-related quality of life and reduce symptoms: which groups benefit most? Acta oncologica (Stockholm, Sweden). 2021;60:403–11. https://doi.org/10.1080/0284186X.2020.1851764.
Nemes S, Jonasson JM, Genell A, Steineck G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009;9:56. https://doi.org/10.1186/1471-2288-9-56.
van Ploos Amstel FK, Peters MEWJ, Donders R, et al. Does a regular nurse-led distress screening and discussion improve quality of life of breast cancer patients treated with curative intent? A randomized controlled trial Psycho-Oncology. 2020;29:719–28. https://doi.org/10.1002/pon.5324.
Hollingworth W, Metcalfe C, Mancero S, et al. Are needs assessments cost effective in reducing distress among patients with cancer? A randomized controlled trial using the distress thermometer and problem list. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:3631–8. https://doi.org/10.1200/JCO.2012.48.3040.
van der Meulen I, May A, Koole R, Ros W. A distress thermometer intervention for patients with head and neck cancer. ONF. 2018;45:E14–32. https://doi.org/10.1188/18.ONF.E14-E32.
Braeken APBM, Kempen GIJM, Eekers DBP, et al. Psychosocial screening effects on health-related outcomes in patients receiving radiotherapy. A cluster randomised controlled trial Psycho-oncology. 2013;22:2736–46. https://doi.org/10.1002/pon.3340.
Mertz BG, Dunn-Henriksen AK, Kroman N, et al. The effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: a randomized pilot study. Acta Oncol. 2017;56:1682–9. https://doi.org/10.1080/0284186X.2017.1358462.
Meijer A, Roseman M, Delisle VC, et al. Effects of screening for psychological distress on patient outcomes in cancer: a systematic review. J Psychosom Res. 2013;75:1–17. https://doi.org/10.1016/j.jpsychores.2013.01.012.
Rankin NM, Butow PN, Thein T, et al. Everybody wants it done but nobody wants to do it: an exploration of the barrier and enablers of critical components towards creating a clinical pathway for anxiety and depression in cancer. BMC Health Serv Res. 2015;15:28. https://doi.org/10.1186/s12913-015-0691-9.
Geerligs L, Shepherd HL, Butow P, et al. What factors influence organisational readiness for change? Implementation of the Australian clinical pathway for the screening, assessment and management of anxiety and depression in adult cancer patients (ADAPT CP). Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2020. https://doi.org/10.1007/s00520-020-05836-9.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Federal Ministry of Health within the funding program of the “National Cancer Plan” (NKP-332–058). The funding source was not involved at any stage of the research process.
Author information
Authors and Affiliations
Contributions
Conceptualization: AMT, UK, LS.
Methodology: AMT, UK, LS, MR, SN, OW.
Investigation: AMT, LS, SS, GS.
Data Curation: LS, MF, PE.
Formal analysis: PE, MF.
Validation: MF.
Resources: GS, CB.
Software: MR, SN, OW.
Project administration: AMT, LS.
Supervision: AMT, UK.
Funding acquisition: AMT, UK.
Visualization: PE.
Writing — original draft: PE, LS.
Writing — review and editing: PE, LS, AMT, UK, GS, CB, MR, MF, SS, SN, OW.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the ethics committee of the medical chamber of Hamburg (PV4371).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients agreed that their data will be published at the group level which does not contain any references to their identity.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esser, P., Sautier, L., Sarkar, S. et al. Evaluation of an electronic psycho-oncological adaptive screening program (EPAS) with immediate patient feedback: findings from a German cluster intervention study. J Cancer Surviv 16, 1401–1413 (2022). https://doi.org/10.1007/s11764-021-01121-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-01121-8