Abstract
Purpose
Although users of aromatase inhibitors have higher total fracture risk in some randomized trials, little is known about their risk outside of clinical trials or in older higher-risk cohorts.
Methods
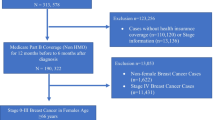
In a population-based retrospective cohort study, we identified all older US Medicare D prescription drug insurance plan-enrolled women who had initial breast cancer surgery in 2006–2008 and began hormonal therapy (an aromatase inhibitor (AI) or tamoxifen) within the subsequent year. Total nonvertebral and hip fractures through 2012 were identified using a validated algorithm. The association of fracture outcomes with hormonal therapy type was assessed using competing risk regression models that accounted for differences in measured baseline covariates. Treatment assignment bias was reduced using inverse probability of treatment weighting computed from propensity scores.
Results
Among 23,378 women taking hormonal therapy (23.2% aged 80 or over), there were 3000 total and 436 hip fractures. Although AI users were younger and had lower comorbidity, after propensity score weighting, these and other covariates were balanced. Total nonvertebral risk was higher for users of AIs compared with tamoxifen, HR 1.11 (1.02–1.21), but the small increase in risk for hip fracture was not statistically significant, HR 1.04 (0.84–1.30).
Conclusions
Although total nonvertebral fracture risk was higher among AI users, differences in hip fractures were not significant in a large population-based cohort of older women.
Implications for Cancer Survivors
Use of aromatase inhibitors by older women is associated with high risk for nonvertebral fracture that is increased compared with use of tamoxifen. Fracture risk should be assessed among patients taking these medications.

Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–52.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2. https://doi.org/10.1016/S0140-6736(04)17666-6.
Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA. Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc. 2012;60(9):1761–7. https://doi.org/10.1111/j.1532-5415.2012.04107.x.
Yen TWF, Czypinski LK, Sparapani RA, Guo C, Laud PW, Pezzin LE, et al. Socioeconomic factors associated with adjuvant hormone therapy use in older breast cancer survivors. Cancer. 2011;117(2):398–405. https://doi.org/10.1002/cncr.25412.
Vaz-Luis I, Keating NL, Lin NU, Lii H, Winer EP, Freedman RA. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32(9):927–34. https://doi.org/10.1200/JCO.2013.51.1261.
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12(12):1101–8. https://doi.org/10.1016/S1470-2045(11)70270-4.
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–19. https://doi.org/10.1056/NEJMoa1604700.
Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–57. https://doi.org/10.1200/JCO.2003.08.017.
Kolata G. Bone diagnosis gives new data but no answers. The New York Times 2003 September 28.
Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39(6 Pt 1):1733–49. https://doi.org/10.1111/j.1475-6773.2004.00315.x.
Neuner JM, Kamaraju S, Charlson JA, Wozniak EM, Smith EC, Biggers A, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Can Inst. 2015;107(8) https://doi.org/10.1093/jnci/djv130.
Neuner JM, Yen TW, Sparapani RA, Laud PW, Nattinger AB. Fracture risk and adjuvant hormonal therapy among a population-based cohort of older female breast cancer patients. Osteoporos Int. 2010;22(11):2847–55. https://doi.org/10.1007/s00198-010-1493-x.
Neuner JM, Zhang X, Sparapani R, Laud PW, Nattinger AB. Racial and socioeconomic disparities in bone density testing before and after hip fracture. J Gen Intern Med. 2007;22:123–45.
Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–14. https://doi.org/10.1016/0895-4356(92)90047-Q.
Curtis JR, Mudano AS, Solomon DH, Xi J, Melton E, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47(1):69–72. https://doi.org/10.1097/MLR.0b013e3181808c05.
Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1(4):E1–E26. https://doi.org/10.5600/mmrr.001.04.a04.
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. https://doi.org/10.1016/S0895-4356(96)00268-5.
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington D.C.: National Osteoporosis Foundation 2014.
Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–6. https://doi.org/10.1200/JCO.2005.02.3028.
Freedman RA, Vaz-Luis I, Barry WT, Lii H, Lin NU, Winer EP, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145(2):491–501. https://doi.org/10.1007/s10549-014-2968-9.
Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Prog Biomed. 2004;75(1):45–9. https://doi.org/10.1016/j.cmpb.2003.10.004.
Fine JP, Gray RJA. Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144.
Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. J Am Med Assoc. 1990;263(5):665–8. https://doi.org/10.1001/jama.1990.03440050059033.
Ligibel JA, O’Malley AJ, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Tr. 2012;131(2):589–97. https://doi.org/10.1007/s10549-011-1754-1.
Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–8. https://doi.org/10.1016/S0140-6736(13)62292-8.
Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. New Eng J Med. 2011;364(25):2381–91. https://doi.org/10.1056/NEJMoa1103507.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Breast Cancer. 2016;
Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–46. https://doi.org/10.1007/s00198-007-0343-y.
Ettinger B, Black DM, Dawson-Hughes B, Pressman AR, Melton LJ. Updated fracture incidence rates for the US version of FRAX®. Osteoporos Int. 2010;21(1):25–33. https://doi.org/10.1007/s00198-009-1032-9.
Dowd B, Maciejewski ML, O’Connor H, Riley G, Geng Y. Health plan enrollment and mortality in the Medicare program. Health Econ. 2011;20(6):645–59. https://doi.org/10.1002/hec.1623.
Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–8. https://doi.org/10.1007/s40471-015-0053-5.
Funding
The study was funded by the American Cancer Society (RSG-11-098-01-CPHPS to JMN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Neuner, J.M., Shi, Y., Kong, A.L. et al. Fractures in a nationwide population-based cohort of users of breast cancer hormonal therapy. J Cancer Surviv 12, 268–275 (2018). https://doi.org/10.1007/s11764-017-0666-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0666-4