Abstract
The inflammatory balance is an important factor in the clinical course of COVID-19 (SARS-CoV-2) infection, which has affected over 300 million people globally since its appearance in December 2019. This study aimed to evaluate the correlation between exhaled nitric oxide (FeNO) level and parenchymal involvement in COVID-19. The study included 106 patients with the delta variant of COVID-19 identified by real-time PCR as well as 40 healthy control groups between October 2021 and March 2022. The patients were analyzed in three groups: moderate COVID-19 (group 1), severe COVID-19 without macrophage activation syndrome (MAS) (group 2), and severe COVID-19 with MAS (group 3). FeNO and CT scores were significantly higher in groups 2 and 3 at admission and discharge compared to group 1 (p = 0.001 for all). In addition, CT score at admission and CT score and FeNO level at discharge were higher in group 3 than in group 2 (p = 0.001 for all). It was found that the FeNO levels were higher in Groups 2 and 3 than in the control group (p = 0.001) during the admission. FeNO and CT scores showed strong positive correlation at admission and discharge (r = 0.917, p = 0.001; r = 0.790, p = 0.001). In receiver operating characteristic curve analysis for prediction of MAS, FeNO at a cut-off of 10.5 ppb had 66% sensitivity and 71% specificity. COVID-19 causes more severe lung involvement than other viral lower respiratory tract infections, leading to the frequent use of chest CT in these patients. FeNO assessment is a practical and noninvasive method that may be useful in evaluating for parenchymal infiltration in the diagnosis and follow-up of COVID-19 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For more than 2 years, the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been the most important health problem in the world. COVID-19 has affected over 300 million people since it first appeared in December 2019. The morbidity and mortality caused by COVID-19 has also started to manifest serious economic and psychological consequences. In particular, the lack of effective anti-viral treatment and spikes in infections with new variants increase the economic burden. While the infection is generally milder in vaccinated individuals, severe clinical presentations such as acute respiratory failure syndrome (ARDS) and macrophage activation syndrome (MAS) are still seen in unvaccinated COVID-19 patients [1].
Studies to predict the clinical course of COVID-19 have shown that numerous proinflammatory cytokines are released, including tumor necrosis factor alpha (TNF-α), interleukins 1, 2, 6, and 18, and nitric oxide (NO). Excessive cytokine release increases vascular permeability and leads to impaired tissue perfusion, endothelial damage, and microthrombus formation. Fluid accumulation in the lung tissue and interstitial spaces due to vascular hyperpermeability manifests clinically as acute respiratory failure. Proinflammatory cytokine suppression is a therapeutic strategy in many inflammatory conditions, including viral infections [2, 3].
Resident and inflammatory cells in the human airways produce NO and related compounds through the oxidation of l-arginine catalyzed by nitric oxide synthases (NOSs) [4]. Of the three NOS isoforms, NOS1 and NOS3 can be secreted by various pulmonary cells, whereas NOS2 is expressed by non-noradrenergic, non-cholinergic fibers [5]. In addition to its important role in smooth muscle relaxation, NO synthesized in the airways can suppresses the replication of a number of DNA and RNA viruses, including respiratory viral strains such as influenza virus, respiratory syncytial virus, rhinoviruses, and coronaviruses [6].
Chest computed tomography is frequently performed to better understand parenchymal involvement in patients with COVID-19. It is even used in staging and follow-up. In this study, we aimed to evaluate the correlation of fractional exhaled NO (FeNO) with disease course and radiological involvement to identify alternative diagnostic methods that may spare patients from the effects of excessive radiation.
Materials and methods
Study design
The study included patients who presented to the emergency department of Erzurum Regional Training and Research Hospital with symptoms consistent with COVID-19 (fever, cough, dyspnea, malaise, sudden loss of taste and smell) and a history of international travel or risky contact within the last 14 days. The patients and/or their relatives were provided detailed information about the study aim and procedures, and all participants provided informed consent before inclusion. Local ethics committee approval was obtained prior to the study. This study was designed and conducted in accordance with the ethical guidelines set forth in the Declaration of Helsinki, and the study protocol was approved by the local ethics committee (B.30.2.ATA.0.01.00/176). This research was supported by the Ataturk University Scientific Research Project Office (project number 9480).
Study population
Standard high-resolution computed tomography (HRCT) was performed for patients considered at high risk of COVID-19. Bilateral, predominantly peripheral ground glass opacities, subsegmental consolidation or linear opacities, crazy-paving pattern, and reverse halo sign were considered HRCT findings typical of COVID-19. Patients with these findings and those showing atypical radiological findings but consistent clinical symptoms were admitted. Real-time polymerase chain reaction (PCR) testing of nasopharyngeal swab samples was performed to confirm the diagnosis of COVID-19. There was a total of 146 participants, 106 of whom did not get vaccinated with inactivated virus and/or mRNA for COVID-19 and 40 patients who had not been vaccinated with inactivated virus and/or mRNA due to COVID-19 who were hospitalized in the intensive care and wards of for chest diseases due to moderate and severe COVID-19 pneumonia between October 2021 and March 2022 in the Erzurum Regional Education and Research Hospital.
Hematological parameters, biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, D-dimer, troponin-I, and C-reactive protein (CRP) levels were evaluated daily starting from the day of admission. The patients' FeNO levels were examined upon admission and discharge.
Study groups
The patients were analyzed in three groups based on COVID-19 severity. Group 1 (n = 24) included patients with moderate illness (non-severe pneumonia), group 2 (n = 26) included patients who presented with severe pneumonia but were not admitted to the intensive care unit due to respiratory failure or MAS, and group 3 (n = 56) included patients who presented with severe pneumonia and were admitted to the intensive care unit due to MAS or respiratory failure. Severe pneumonia was diagnosed in patients meeting any of the following criteria: respiratory rate ≥ 30 breaths/min, SpO2 ≤ 92%, and > 50% lung infiltration rate. Control Group: A healthy control group (n:40) who had not previously received any inactivated viral or mRNA vaccine, who had admitted to our vaccination clinic for their first dose of vaccine, and who did not get COVID-19.
FeNO measurement
FeNO was measured according to the 2011 American Thoracic Society (ATS) recommendations prior to spirometry so as not to impact the results [7]. Measurements were made using a NObreath® portable analyzer (Bedfont, Kent, UK), in which the expiratory flow is maintained at 50 mL/s and is controlled by an acoustic emission signal. To reduce the effects of the circadian cycle, the FeNO value was tested at the same time upon discharge as measured during hospitalization.
Exclusion criteria
In line with the recommendations in the ATS 2011 guideline, factors that may affect FeNO levels were evaluated. Smoking, nitrates in food and beverages, alcohol, and active exercise before the test may affect FeNO readings. Therefore, we confirmed that patients had not done any of these within 1 h before the test. Patients receiving NO synthesis inhibitors, oral or intravenous corticosteroid therapy, or oral, inhaled, or intravenous l-arginine were excluded from the study. In addition, patients with any of the following conditions that affect FeNO level were not included: asthma, chronic obstructive pulmonary disease, cystic fibrosis, diagnosed pulmonary hypertension, HIV infection, bronchiectasis, systemic lupus erythematosus, acute organ resection, and post-transplantation bronchiolitis obliterans.
Chest computed tomography imaging and analysis
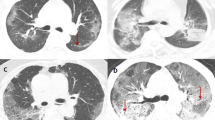
Low-dose thoracic tomography was conducted upon admission to the hospital as well as on the 14th day of hospitalization, which is the day of the patient's discharge. All patients underwent contrast-enhanced CT scans of the chest on a second-generation Somatom Definition Flash 256-slice dual-source multidetector CT scanner (Siemens Healthcare, Forchheim, Germany). CT examinations were performed with breath holding during deep inspiration. All images were transferred to a commercial workstation (Singo via. Workstation, Siemens, Erlangen, Germany) and assessed by two pulmonologists blinded to the patients’ identities. The first and second readers had 20 and 15 years of experience in pulmonology, respectively. Each reader independently evaluated the size, location, and number of the lesions and interpreted the findings.
In tomographic imaging, we used the international standard terminology defined by the Fleischner Society dictionary and terms such as ground-glass density, crazy-paving pattern and consolidation defined in the literature on viral pneumonia [8,9,10]. A semi-quantitative scoring system was used to quantitatively estimate the pulmonary involvement based on the total area involved by all detected abnormalities [11]. Each of the five lung lobes was visually scored as 0 (no involvement), 1 (< 5% involvement), 2 (25% involvement), 3 (26–49% involvement), 4 (50–75% involvement), or 5 (> 75% involvement). Total CT score was calculated as the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement) [12].
Definitions and diagnosis
Fever was defined as an axillary temperature of 37.3 °C or higher. ARDS was diagnosed and graded according to the Berlin 2015 diagnostic criteria [13]. Coagulopathy was defined as prolonged prothrombin time (3 s longer than normal) and partial thromboplastin time (5 s longer than normal). Patients with elevated cardiac-specific troponin levels underwent echocardiographic evaluation for cardiac pathology.
COVID-19 treatment protocols were determined individually based on clinical severity as specified in the Turkish Ministry of Health COVID-19 adult diagnosis and treatment guidelines [14]. Patients with high fever during treatment for COVID-19 were given empiric antibiotherapy and tested for possible bacterial and fungal superinfections with blood, urine, and sputum cultures. Antibiotherapy was adjusted according to culture results. Patients hospitalized with moderate COVID-19 were therefore treated with 2–4 l/min nasal oxygen therapy along with 6 mg/day dexamethasone medication for 7 days. As an antiviral treatment, favipiravir was administered for 5 days at a dosage of 2 × 1600 mg as loading dose and 2 × 600 mg as maintenance dose. Alongside to the treatment of patients hospitalized with moderate COVID-19 pneumonia, oxygen therapy was delivered via a high-flow nasal cannula with SpO2 > 92% to the hospitalized patients with severe COVID-19 pneumonia. With the improvement in saturation levels, nasal oxygen therapy of 2–4 l/min was initiated.
MAS was suspected in patients exhibiting findings such as refractory fever, persistently high or increasing CRP and ferritin levels, elevated D-dimer level, cytopenia (lymphopenia or thrombocytopenia), abnormal liver function tests, hypofibrinogenemia, and elevated triglyceride levels despite treatment. If serial measures demonstrated further deterioration in these parameters that could not be explained by secondary bacterial infection, > 250 mg/day methylprednisolone was administered as treatment for MAS if the patient had no contraindication. Patients who showed no clinical response after 72 h were treated with 400-mg tocilizumab. Those who still showed no clinical and laboratory response after 24 h received a second dose of tocilizumab.
Statistical analysis
IBM SPSS version 20.0 software (IBM Corp, Armonk, NY) was used for statistical analyses. Data were presented as mean, standard deviation, number, and percentage. Shapiro–Wilk test and Kolmogorov–Smirnov test were used to determine whether continuous variables were normally distributed. Continuous variables were compared between more than two independent groups using analysis of variance (ANOVA) if normally distributed and Kruskal–Wallis test if non-normally distributed. Post hoc tests after ANOVA were performed using Tukey’s test when variances were homogeneous and Tamhane’s T2 test when variances were not homogeneous. Kruskal–Wallis one-way ANOVA (k samples) test was used as a post hoc analysis after the Kruskal–Wallis test. Pearson and Spearman correlation analyses were used to examine relationships between normally and non-normally distributed quantitative variables, respectively. Receiver operating characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of selected variables in discriminating patients with severe COVID-19 requiring intensive care. Statistical significance was accepted at p < 0.05.
Results
The average age of the participants in our study was 58.9 ± 15.8 years. The average age of patients in Group 1 was 57.2 ± 17.2, in Group 2, it was 59.7 ± 11.4, and in Group 3 it was 60.3 ± 15.9. The control group had an average mean age of 58.9 ± 13.2 years. There was no statistically significant difference between the average patient age and the average age of the control group (p = 0.53). Sixty (56.6%) participants involved in the study were male, compared to 40 (62.5%) participants in the control group. There was no statistically significant variation in the distribution of the groups by gender (p = 0.21). While 30 of the patients were former smokers, 76 were nonsmokers. Of the patients, 45 of them were diagnosed with hypertension, 21 with diabetes mellitus, and 5 with coronary artery disease. Twenty patients in the control group were former smokers, 15 had hypertension, 12 had diabetes mellitus, and 2 had coronary artery disease.
Analysis of the patients’ laboratory parameters during hospitalization is shown in Table 1. Lymphocyte count and percentage were significantly lower in groups 2 and 3 compared to group 1 (p = 0.001, 0.04). In addition, CRP, lactose dehydrogenase (LDH), blood urea nitrogen (BUN), ferritin, and aspartate transaminase (AST) were higher in groups 2 and 3 compared to group 1, and CRP was also higher in group 3 than in group 2.
Table 2 displays the FeNO and CT scores of the patients during hospitalization and at the time of discharge, as well as those of the control group. FeNO and CT scores were significantly higher in groups 2 and 3 both at admission and discharge compared to group 1 (p = 0.001 for all), while CT score at admission and both CT score and FeNO level at discharge were higher in group 3 than in group 2 (p = 0.001 for all). While the FeNO levels of Groups 2 and 3 were statistically significantly higher than those of the control group at the time of hospitalization (p = 0.001 for both groups), only the FeNO levels of Group 3 patients were statistically significantly higher than those of the control group at the time of discharge (p = 0.02, 0.001, respectively).
The correlation analysis between FeNO and selected parameters at time of admission is shown in Table 3. FeNO was positively correlated with CT score, LDH, and CRP, with the strongest correlation to CT score (r = 0.917, p = 0.001, r = 0.780, p = 0.001, r = 0.491, p = 0.001). Figure 1 shows a graphical representation of the patients’ FeNO and CT scores at admission and discharge. The positive correlation between FeNO and CT score persisted at discharge, though it was not as strong (r = 0.790, p = 0.001).
ROC curve analysis of LDH, CRP, FeNO, and CT scores at admission in patients who developed severe pneumonia and MAS and were treated in the intensive care unit compared to those who did not require intensive care is shown in Fig. 2. At a cut-off value of 10.5 ppm, FeNO showed 66% sensitivity and 71% specificity in discriminating between these two groups. At a cut-off value of 6.5 for CT score, sensitivity was 60% and specificity was 81%. Cut-off values of 19.5 mg/dl for CRP and 308.5 U/L for LDH had sensitivity values of 80% and specificity values of 79% and 65%, respectively.
Discussion
Chest CT provides more valuable information than chest X-ray for the evaluation of parenchymal involvement in patients with COVID-19 pneumonia. In this present study, we observed that FeNO levels assessed during hospitalization increased in correlation with pulmonary parenchymal involvement detected on CT. Cytokine storm is known to have an important role in the course of COVID-19. We also observed in this study that presenting FeNO level was higher in patients with clinical manifestations of cytokine storm (ARDS and MAS) compared to patients with moderate and severe COVID-19 who did not develop cytokine storm. The difference became less pronounced but was still present at discharge.
Much has been learned about the clinical course of COVID-19 over the last 2 years, but there are still aspects of the disease that remain a mystery. Many patients with COVID-19 never develop hypoxemia and respiratory distress, but comorbid and unvaccinated patients often present a more severe course and patients with no respiratory distress at presentation often progress to MAS and ARDS [15]. Although PCR testing of a nasopharyngeal sample is still regarded as the principal diagnostic method for COVID-19, studies have shown that the high sensitivity of thoracic CT used to detect lung involvement in COVID-19 patients may be more valuable than PCR testing [16]. However, the extensive use of chest CT is an economic burden and a cause of unnecessary radiation exposure for patients. The use of lung X-ray, which is a safer and more readily accessible method for the follow-up of these patients, as well as proinflammatory markers such as CRP, fibrinogen, D-dimer, and ferritin has been shown to be important in treatment planning and prognosis [2].
FeNO is another important proinflammatory biomarker used to indicate airway inflammation in the follow-up of asthma patients [17]. NO is an endogenously produced chemical that can be a marker and mediator of inflammatory diseases affecting the pulmonary system. Studies have shown that FeNO levels increase with acute exacerbations and decrease with treatment in asthma, as well as COPD, cystic fibrosis, Sjögren’s syndrome, and some other chronic lung diseases. In studies investigating the effect of viral infection on FeNO levels in patients with asthma, rhinovirus infection was found to increase FeNO levels, and this increase was negatively associated with worsening of airway hypersensitivity to histamine. These results suggest that viral induction of NOS in the airways may play a protective role in asthma exacerbations [18]. FeNO is strongly related to the type-2 inflammatory response found in asthma, which has been suggested to be protective against SARS-CoV-2 infection. In addition, treating COVID-19 patients in the intensive care unit with NO was reported to bring about faster improvement in the ventilation-perfusion equilibrium and reduce the need for mechanical ventilation [19]. In another study involving post-COVID-19 patients, it was discovered that patients with ground-glass opacity and fibrotic band formations had a greater FeNO level than patients in the control group [20].
In our study, we observed lower lymphocyte levels and higher CRP, LDH, and ferritin levels with increasing COVID-19 severity, consistent with previous studies [3, 21, 22]. Like many other viral infections, COVID-19 is lymphotropic and can cause severe endothelial and epithelial damage. This may largely explain our findings in the present study. Assessment of FeNO levels demonstrated an increase correlated with parenchymal infiltration both at admission and discharge, and this correlation was also consistent with laboratory parameters known to be important markers in the follow-up of COVID-19, such as LDH and CRP. COVID-19 can cause more severe involvement of the lung tissue compared to other viral infections. An increase in the anti-inflammatory molecule NO to balance this inflammatory effect may have resulted in higher FeNO levels. The correlation of FeNO with CRP and LDH levels at admission can also be interpreted as confirmation of this. No difference was detected between the moderate Covid-19 patients and the control group, whereas a greater FeNO level was observed for severe COVID-19 patients. Moreover, the FeNO level at discharge was lower in moderate COVID-19 patients compared to the control group, whereas it remained higher in patients with severe COVID-19 and MAS is developed, despite a drop from baseline was observed. This result can be evaluated mostly as a result of moderate COVID-19 individuals receiving steroid medication in addition to oxygen therapy. Additionally, high-dose steroid medication and anti-cytokine medicines used on individuals with severe COVID-19 may have caused to a decrease along with oxygen therapy. However, even if a decline is found in patients with MAS, the high FeNO level relative to the control group can be interpreted as a reduction in inflammation has been continuing. As the medical treatment given to the patients during follow-up varied according to disease course as per our national guideline and this may have differentially affected FeNO levels, we did not evaluate the correlation between FeNO and laboratory data at discharge in this study. In the ROC curve analysis, FeNO and CT scores had low sensitivity compared to CRP and LDH in the prediction of disease severity. This may be attributable to the appearance of parenchymal progression over time and the short half-life of FeNO. Compared to prior studies of post-COVID-19 patients, lower FeNO was observed in both the patient and control groups. This may be due to the influence of altitude in the two studies. Studies assessing the influence of altitude on NO partial pressure indicate that lower NO levels can occur in exhaled air at higher altitudes [23]. The distance between the post-COVID-19 patients and the study carried out in our city is roughly 1900 m.
One of the limitations of our study was the inability to exclude the possible effect of sex on FeNO. Although no significant difference in sex distribution was found between our patient groups, studies in which this factor can be completely ruled out may yield more reliable results.
In conclusion, CT findings have provided important information in the diagnosis and follow-up of COVID-19 since the start of the pandemic. However, this imaging modality is associated with radiation exposure and is not recommended for routine follow-up. FeNO, which is primarily used to assess parenchymal inflammation in patients with asthma, has been shown to be effective in the follow-up of viral lower respiratory tract infection. Our results indicate that when evaluated in correlation with laboratory parameters, this practical and noninvasive method may be useful both in predicting clinical course at the time of admission and during follow-up.
References
Uzun O, Akpolat T, Varol A, Turan S, Bektas SG, Cetinkaya PD, Dursun M, Bakan N, Ketencioglu BB, Bayrak M (2022) COVID-19: vaccination vs. hospitalization. Infection 50(3):747–752
Kerget B, Kerget F, Koçak AO, Kızıltunç A, Araz Ö, Uçar EY, Akgün M (2020) Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID-19? Lung 198(5):777–784
Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M (2021) Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol 93(4):2090–2098
Stuehr DJ (2004) Enzymes of the l-arginine to nitric oxide pathway. J Nutr 134(10):2748S-2751S
Gao P, Kawada H, Kasamatsu T, Mao XQ, Roberts MH, Miyamoto Y, Yoshimura M, Saitoh Y, Yasue H, Nakao K (2000) Variants of NOS1, NOS2, and NOS3 genes in asthmatics. Biochem Biophys Res Commun 267(3):761–763
Gadish T, Soferman R, Merimovitch T, Fireman E, Sivan Y (2010) Exhaled nitric oxide in acute respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med 164(8):727–731
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin A-C, Plummer AL, Taylor DR, Applications ATSCoIoENOLfC (2011) An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184(5):602–615
Franquet T (2011) Imaging of pulmonary viral pneumonia. Radiology 260(1):18–39
Koo HJ, Lim S, Choe J, Choi S-H, Sung H, Do K-H (2018) Radiographic and CT features of viral pneumonia. Radiographics 38(3):719–739
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697–722
Chang Y-C, Yu C-J, Chang S-C, Galvin JR, Liu H-M, Hsiao C-H, Kuo P-H, Chen K-Y, Franks TJ, Huang K-M (2005) Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology 236(3):1067–1075
Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 295(3):715–721
Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ (2018) Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest 153(2):361–367
Bakanliği TS (2020) COVID-19 (SARS-CoV2 Enfeksiyonu) Rehberi. Erişim (Erişim Tarihi: July 16, 2020)
Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: a review. Clin Immunol 215:108427
Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Li Z, Li X, Li H (2020) Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 126:108961
Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G (2004) Nitric oxide in health and disease of the respiratory system. Physiol Rev 84(3):731–765
De Gouw H, Grunberg K, Schot R, Kroes A, Dick E, Sterk P (1998) Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J 11(1):126–132
Ricciardolo FLM, Bertolini F, Carriero V, Högman M (2020) Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J Breath Res 15(1):014001
Cameli P, Bargagli E, Bergantini L, d’Alessandro M, Giugno B, Gentili F, Sestini P (2021) Alveolar nitric oxide as a biomarker of COVID-19 lung sequelae: a pivotal study. Antioxidants 10(9):1350
Kerget B, Kerget F, Aksakal A, Aşkın S, Uçar EY, Sağlam L (2021) Evaluation of the relationship between KIM-1 and suPAR levels and clinical severity in COVID-19 patients: a different perspective on suPAR. J Med Virol 93(9):5568–5573
Payán-Pernía S, Gómez Pérez L, Remacha Sevilla ÁF, Sierra Gil J, Novelli Canales S (2021) Absolute lymphocytes, ferritin, C-reactive protein, and lactate dehydrogenase predict early invasive ventilation in patients with COVID-19. Lab Med 52(2):141–145
Ghosh S, Kiyamu M, Contreras P, León-Velarde F, Bigham A, Brutsaert TD (2019) Exhaled nitric oxide in ethnically diverse high-altitude native populations: a comparative study. Am J Phys Anthropol 170(3):451–458
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors received no financial support for the research and/or authorship of this article. The authors declare that they have no conflict of interest to the publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The article belongs to COVID 19.
Rights and permissions
About this article
Cite this article
Kerget, B., Araz, Ö. & Akgün, M. The role of exhaled nitric oxide (FeNO) in the evaluation of lung parenchymal involvement in COVID-19 patients. Intern Emerg Med 17, 1951–1958 (2022). https://doi.org/10.1007/s11739-022-03035-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03035-4