Abstract
Previous research yielded conflicting results on the association between cigarette smoking and risk of SARS-CoV-2 infection. Since the prevalence of smoking is high globally, the study of its impact on COVID-19 pandemic may have considerable implications for public health. This study is the first to investigate the association between the SARS-CoV-2 antibody sero-positivity and biochemically verified smoking status, to refine current estimates on this association. SARS-CoV-2-specific IgG and serum cotinine levels (a well-known marker of tobacco exposure) were assessed in a large sero-epidemiological survey conducted in the town of Troina (Sicily, Italy). A propensity score matching was carried out to reduce the effect of possible factors on SARS-CoV-2 infection risk among study participants. Of the 1785 subjects included in our study, one-third was classified as current smokers, based on serum cotinine levels. The overall proportion of subjects with positive serology for SARS-CoV-2 IgG was 5.4%. The prevalence of SARS-CoV-2 antibody positivity and previous COVID-19 diagnosis were reduced in smokers. This reduced prevalence persisted after adjusting for possible confounders (such as sex, age, previous infection, chronic conditions, and risk group) at regression analyses, and the point estimates based on the PS-matched models resulted consistent with those for the unmatched population. This study found a lower proportion of positive SARS-CoV-2 serology among current smokers, using direct laboratory measures of tobacco exposure and thus avoiding possible bias associated with self-reported smoking status. Results may also serve as a reference for future clinical research on potential pharmaceutical role of nicotine or nicotinic-cholinergic agonists against COVID-19.
Similar content being viewed by others
Introduction
The severe acute respiratory syndrome—coronavirus 2 (SARS-CoV-2) pandemic represents a major public health challenge, having resulted in almost 395 million confirmed infections and 5.7 million deaths reported to the World Health Organization in the last 2 years, with relative societal and healthcare system demands [1,2,3].
Since the spread of the first coronavirus disease 2019 (COVID-19) cases—the disease associated with SARS-CoV-2 infection—in late 2019, the pandemic has spotlighted the essential need for research efforts towards understanding the clinical features of the disease, as well as to develop treatments and vaccines to tackle this health emergency [4,5,6]. As the global burden of COVID-19 continues to rise, observational evidence is focusing on the study of possible predictors and risk factors for SARS-CoV-2 infection and COVID-19 outcomes [5, 7,8,9,10,11,12].
Approximately 1.3 billion people worldwide use tobacco, of which more than 7 million die prematurely every year [13]. Smoking is the main cause of lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease [14,15,16].
Smoking is also a significant risk factor for both viral and bacterial infections of the respiratory system [17, 18], and the association between smoking and COVID-19 has caught the attention of the research community from the very first epidemic weeks [19, 20].
Surprisingly current research on the relationship between smoking and SARS-CoV-2/COVID-19 has yielded conflicting findings [19,20,21]. For instance, a positive association between smoking and risk of COVID-19-related severe outcomes, hospitalization and death was found in some primary and secondary studies [22,23,24,25]. In contrast, other studies have identified lower proportions of active smokers among patients diagnosed with SARS-CoV-2 [26,27,28,29], and a significant lower prevalence of smoking among hospitalized COVID-19 patients than that expected on the basis of population smoking prevalence [30, 31]. On both hypotheses, the literature so far available is affected by several design issues, which make studies hard to compare [30,31,32]. In particular, many reports derived data on smoking from case-series, where the chance of inaccurate recording of smoking status/history, or other recall/reporting bias cannot be excluded, in consideration of the challenging circumstances in which data were collected [19, 33]. Recently, Farsalinos et al. looked at a possible systematic ascertainment bias in determining the overall smoking-related risk across the research that reported a higher risk for severe COVID-19 in hospitalized smokers [20]. These authors suggested that, if smokers might be less likely to develop the infection or severe disease [27,28,29], a higher risk for adverse outcome among hospitalized smokers is not applicable to all smokers but only to the small proportion of smokers who end up being hospitalized due to COVID-19 [20].
Additional concerns have been raised about the possible sources of residual confounding. Some studies relied on self-reported smoking habits and most failed to correct for relevant confounders which could result in different risk exposure while others combined current smokers and former smokers into one category, preventing from examining the interaction of SARS-CoV-2 with active smoking and its effect on risk of infection and COVID-19 outcomes [11, 22, 24, 34]. As a whole, all these limitations reflect problems with poor reporting of the smoking status as well as lack of studies specifically designed to examine the association between smoking and COVID-19. All these factors could affect the conclusions derived from the existing body of evidence [19].
To address potential bias in estimation of smoking prevalence by self-reporting, we designed a sero-prevalence study—an attempt to measure the true infection rates in selected populations [26, 35]—to investigate the association between the SARS-CoV-2 antibody sero-positivity and biochemically verified smoking. Serum cotinine measurements were used, which is a well-known marker of tobacco exposure since cotinine is the predominant metabolite of nicotine, and cotinine assays are generally used to discriminate active smokers from non-smokers [36].
Materials and methods
Study design, setting, and population
The Troina project defines a cohort study conducted between July and September 2020. The complete study protocol has been previously published [33]. Surveillance of antibody sero-positivity and biochemically verified smoking status (i.e., cotinine) was conducted in serum samples of the general population and healthcare workers (HCW) from the town of Troina (Province of Enna, Sicily, Italy). Troina has a population of over 9000 inhabitants and in March 2020 censused a high incidence of SARS-CoV-2 infections during the early weeks of the first wave of the pandemic, prompting local authorities to declare the town a ‘red zone’ and to enforce lockdown rules on March 29, although no relevant difference in hospitalization and mortality rates was registered compared to regional data [33, 37].
The study population consisted of two groups: (i) a population-based, gender and age-stratified cohort randomly selected from the population registry of town residents; (ii) a convenience sample of hospital care workers (HCW) of Troina’s main health facility (IRCCS Oasi Maria Santissima, Troina, Enna, Italy), who were likely to have been in close contact with COVID-19 patients and, therefore, at high risk of infection. Any individual, irrespective of age, who lived in the town of Troina or worked in the selected institution was eligible for inclusion in the study. Subjects who refused to provide informed consent or had contraindication to venepuncture were excluded. Suspected or confirmed active/acute, recent, or prior SARS-CoV-2 infection was not considered as an exclusion criterion for this investigation. For individuals receiving medical care for COVID-19 during the study period, a proxy respondent (i.e., family member) was contacted to gather questionnaire responses.
The cohort assembly and sample size determination followed the WHO protocol for sero-epidemiological investigation for SARS-CoV-2 infection [38], and has been described in detail previously [33]. Briefly, to aim for a representative sample of the population by gender and age groups, a targeted sample size for this study was specified for each category. We, therefore, calculated several sample sizes depending on the margin of error equal or minor than 3% for estimate proportions of sampled population. Consequently, a sample of subjects was sought in the entire population. The attrition rate was fixed at 10%. In the second group, all workers from the selected institution were invited to participate in the study. Participation was voluntary; enrolees were not offered any incentive for and were informed about their right to drop out of the study at any time for any reason or no reason at all, without penalty. Each participant received complete information about the nature and protocol of the research, and informed that all information gathered would be anonymous and confidentiality would be maintained by omitting any personal identifying information. All participants provided informed consent at the enrolment; in case of participants aged less than 18 years, consent was obtained from their parents.
The Research Ethics Committee of the IRCCS Oasi Maria Santissima (Troina, Enna, Italy) approved the research protocol, survey instruments, and informed consent form (approval n. 11/2020).
Study endpoints and data definitions
This study was conducted to: (i) measure the serum concentration of anti-SARS-CoV-2 immunoglobulin classes G (IgG) in the study sample, to quantify the prevalence of subjects with altered immunologic profile due to SARS-CoV-2 infection since the beginning of the pandemic; (ii) evaluate the level of serum cotinine, to quantify the proportion of biochemically verified current, former and never smokers; (iii) analyse the relationship between active smoking (current vs. non-smokers) and SARS-CoV-2 infection. Secondary outcomes included the assessment of: (i) the proportion of participants with of COVID-19-like symptoms; (ii) the prevalence of SARS-CoV-2 confirmed diagnosis in the study sample; (iii) the proportion of subjects hospitalized due to COVID-19; (iv) recording relevant clinical confounders known to be associated with SARS-CoV-2 infection risk and COVID-19 outcomes (including sex, age, occupational exposure to SARS-CoV-2). Smoking was defined as any use of tobacco cigarettes, cigars and/or rolls. Current smokers were defined as those who reported active smoking and with serum cotinine levels ≥ 20 ng/mL; former smokers were those who reported that they smoked in the past, but not at the time of the survey and had serum cotinine levels < 20 ng/mL; never smokers those who reported that they never smoked and had serum cotinine levels < 20 ng/mL [39].
Study procedures
Details about participants’ interviews and blood specimen handling (collection, transport, aliquoting, biobanking, storage, and assays) have been previously described [33]. In brief, a one-site testing-point was set up with trained personnel, where participants were interviewed regarding demographics and professional characteristics (sex, age, working status), health status (presence of comorbidities, use of medications), history of smoking and smoking habits, history of symptoms compatible with COVID-19 (i.e., fever, severe tiredness, sore throat, cough, shortness of breath, headache, anosmia, ageusia, nausea, vomiting, diarrhoea, or any other COVID-19-like symptom), previous diagnosis with SARS-CoV-2 and/or need of medical contacts or hospitalization due to COVID-19. Subsequently, collection of a blood sample (10 mL) by venepuncture was performed on each participant upon at the same testing-point. For each specimen, the time of collection, the conditions for transportation and the time of arrival at the study laboratory were recorded. Specimens reached the laboratory as soon as possible after collection, where serum was separated from whole blood and stored at − 80 °C until use.
Serum samples were screened for anti-SARS-CoV-2 human antibodies using the EUROIMMUN Anti-SARS-CoV-2 Assay, an enzyme-linked immunosorbent assay (ELISA) that provides semi-quantitative in vitro determination of neutralizing IgG that bind the SARS-CoV-2 spike (S) protein receptor binding domain (RBD)—the most critical target for SARS-CoV-2-specific immunoglobulin within the S1 sub-unit [40]. According to the manufacturer’s recommendations, positivity was intended a ratio equal to or greater than 1.1 [41]. The EUROIMMUN Anti-SARS-CoV-2 ELISA test presented sensitivity of 100% (95% CI 91.6–100) and specificity of 97.7% (95% CI 91.9–99.6) four days after COVID-19 diagnosis by real-time polymerase chain reaction (PCR) [42].
For the evaluation of cotinine, pre-conditioned samples were injected into HP-5 Capillary GC Column (0.32 mm ID, 25 m length and 0.52 μm film thickness; bonded 5% phenyl and 95% dimethylpolysiloxane) of GC-NPD. Complete procedures can be found in the study protocol [33]. Serum levels of cotinine were reported in ng/mL. The limit of quantification of cotinine with GC-NPD is 20 ng/mL. The 20 ng/mL value is a very reasonable cut-off for serum cotinine to distinguish smokers from non-smokers [39].
Statistical analysis
Continuous variables were expressed as mean (standard deviation, SD); categorical variables were described as number and percentage. Differences between continuous variables were evaluated though Student t test or Mann–Whitney U test according to their distribution; the chi-squared (χ2) test and Fisher exact test to assess differences among categorical variables. Comparisons were examined among subjects who tested negative and positive to SARS-CoV-2 antibodies. Considering the imbalance of the covariates across smokers’ groups, we performed a propensity score matching (PSM), accounting for those characteristics that were likely to have had an effect on the risk of SARS-CoV-2 infection. The following variables were selected: age, sex, presence of comorbidities (at least, one important chronic condition), cohort group (as proxy of exposure risk to SARS-CoV-2). The propensity score for smoking was calculated using the logistic regression model. According to optimal PSM match ratio and calliper widths for the estimation of differences in means and proportions in observational studies [43, 44], we matched the respondents on a 1:1 ratio, using the nearest neighbouring method with a calliper matching of 0.2. Complete PSM methodology is presented in the Supplementary material.
Subsequently, we fitted multinomial logistic regression analyses to model the association between the outcomes of interest and active smoking, adjusting for possible covariates (age, sex, presence of at least one comorbidity, exposure group, and presence of COVID-19-like symptoms) both in the unmatched and matched study cohorts. The following models were constructed: likelihood of testing positive at SARS-CoV-2 serology (Model 1); likelihood of having received the diagnosis of SARS-CoV-2 infection (Model 2); likelihood of being hospitalized due to COVID-19 (Model 3). The latter, due to the small number of observations which did not allow at making inference on the asymptotic results as in logistic regressions, was built as an exact logistic regression for small samples, in which the log odds of the outcome is modelled as a combination of the predictor variables [45]. Results were reported as adjusted odds ratios (aOR) with 95% confidence intervals (CI). Based on similar approaches in the literature [46], a multivariate Poisson regression analysis with log-link was conducted to model the incidence of COVID-19-like symptoms, as a function of baseline participant characteristics and smoking status. This enabled the relative risks (as incidence rate ratios, RR) associated with a set of covariates to be estimated, including age, sex, and presence of comorbidities. All p values were two-sided and < 0.05 assumed as statistically significant. Statistical analyses were conducted with statistical software STATA version 17 (StataCorp. 2021, College Station, TX, USA) and R version 3.6.2 (R Project for Statistical Computing, Vienna, Austria).
Results
Study population
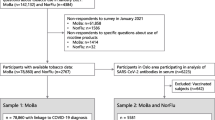
After validating the self-reported smoking status with the serum cotinine threshold to distinguish smokers from non-smokers, a total of 1785 cotinine-verified subjects entered the study: 1312 (73.5%) subjects were sampled from the town of Troina; while 473 (26.5%) constituted the HCW population enrolled in the Troina’s main health care facility. The flowchart of the cohort creation is presented as Fig. 1. Specifically, the majority of participants was female (61.4%), with a mean age of 50 years, and 56.1% had at least one chronic disease. The baseline characteristics of the study population are presented in Table 1, stratified by enrolment group.
SARS-CoV-2 infection and smoking
The overall proportion of subjects with positive serology for SARS-CoV-2-specific IgG was 5.4%. Respondents’ characteristics according to the positivity at antibody testing are listed in Table 2. No significant sex or age differences in antibody response were observed. As expected, HCWs showed a higher prevalence of IgG positivity than general population (72.9 vs. 27.1%, p value < 0.001). A difference in positive serology rate among those who experienced at least one COVID-19-like symptom after March 1, 2020 and those who did not was also present (65.6 vs. 11.4%; p value < 0.001); the found increased rate was confirmed for each of the reported symptoms (p value < 0.001 for all).
Almost one-third the participants smoked (30.4%), while 1242 (69.6%) were classified as former or never smokers, based on the serum cotinine levels. Concordance between self-reported smoking history and serum cotinine threshold was very high, with 97.1% former smokers and 98.7% never smokers having less than 20 ng/mL cotinine level. As regards the relationship between SARS-CoV-2 infection and smoking, the prevalence of SARS-CoV-2 IgG positivity was significantly lower in current smokers (19.8%) than comparators (31.0%, p value = 0.02). No statistically significant association was observed between SARS-CoV-2 IgG positivity and smoking duration. Smokers had higher probability of reporting fever or chills, cough, tiredness, muscle or joint pain, burning throat, and nasal congestion (Table S1, Supplementary materials). Smokers were also more likely to seek medical care because of COVID-19-like symptoms (Table S1, Supplementary materials). Adjusting for covariates at multivariable Poisson regression, tobacco use was associated with a higher incidence rate of COVID-19-like illness, measured as the probability of having experienced at least one symptom (RR 2.45; 95% CI 1.95–3.08; p value < 0.001) (Table S2, Supplementary materials).
Propensity score matching
A propensity scores for smoking status was calculated, accounting for those characteristics that were likely to have had an effect on the risk for SARS-CoV-2 infection and the imbalance of these covariates across groups. The selected characteristics included age, sex, presence of comorbidities (at least, an important chronic condition), and cohort group (as proxy of exposure risk to SARS-CoV-2). The 1:1 matching resulted in 543 matched pairs and a sample size of 1086 patients, with differences between smokers and non-smokers no longer significant in the PS-matched sample. Descriptive statistics before and after PSM, and distributions of propensity scores in smoker and comparison groups’ overlap are, respectively, in Table 3 and Fig. S1 (see Supplementary materials). Compared with before PSM, standardized group differences across all covariates were less than 0.1, representing negligible differences across age, sex, presence of comorbidities, and cohort group (Fig. S2).
Multivariate analyses after PSM
Table 4 shows the OR and 95% CI results for the logistic multivariate regression models. After PSM, Model 1 was designed to analyse the relationship between smoking and positive testing at SARS-CoV-2 serology, indicated that current smoking was associated with a decreased risk of IgG positivity (OR 0.23; 95% CI 0.12–0.045; p value < 0.001). An elevated risk was observed in HCWs (OR 5.45; 95% CI 2.54–11.70; p value < 0.001), in those with at least one chronic condition (OR 2.21; 95% CI 1.13–4.33; p value = 0.02), and in those who reported at least one COVID-19-like symptom (OR 13.38; 95% CI 6.72–26.64; p value < 0.001). As shown in Model 2 (Table 4), the adjusted odds of a diagnosis of SARS-CoV-2 infection by serology testing was significantly lower in smokers (OR 0.51; 95% CI 0.28–0.93; p value = 0.03), but markedly higher in HCW (OR 14.56; 95% CI 6.36–33.30; p value < 0.001). The final model of the multivariate exact logistic regression analysis examining the variables associated with the likelihood of hospitalization due to COVID-19 did not yield significant results (Table 4, Model 3).
Discussion
Our research is the first population-based study that used direct laboratory measures of smoking exposure, aiming at refining the association between active smoking and SARS-CoV-2 infection susceptibility. In this study, we observed a lower proportion of positive SARS-CoV-2 serology in current smokers compared with non-smokers/ex-smokers. Similarly, current smokers were less likely to have received a diagnosis of SARS-CoV-2 infection. No evidence was found about the risk of hospitalization in COVID-19 patients, likely because of the small number of hospitalized cases in our sample. We also conducted logistic regression analyses and found that the association was persistently negative even after adjusting for sex, age, previous SARS-CoV-2 infection, presence of comorbidities, and group of enrolment (as a proxy of infection risk exposure). Furthermore, the point estimates based on the PS-matched models were consistent with those for the whole study population.
Total sero-prevalence of IgG antibodies in this study was 5.4%. This proportion is in line with the results gathered in other population-based sero-epidemiological surveys conducted after the first epidemic wave in the most affected areas in Italy and elsewhere (March–June 2020), also reflecting the significant SARS-CoV-2 circulation in the Troina area [26, 47,48,49,50]. In particular, our study revealed a proportion of subjects with circulating antibodies higher than that detected by the Italian Institute of Statistics (ISTAT) during the same period, although remained below the 7.5% registered in Lombardy region in the same survey, which was hit hardest in terms of cases and death toll during between March and June 2020 [10, 26, 37]. Stratifying by cohort, the rate of sero-prevalence among HCWs peaked at 14.8%, roughly in line with similar studies that surveyed HCWs [37, 51,52,53]. In this regard, it should be mentioned that antibody prevalence in HCWs showed a high variability, according to different aspects of surveys design and conduction, including magnitude of SARS-CoV-2 spread in study settings, type of healthcare facilities and workers enrolled, local availability of personal protective equipment [26].
The reduced risk for confirmed SARS-CoV-2 infection in tobacco users has been previously reported. For instance, Israel described a risk reduction in current smokers [28], and a study conducted in Lombardy region confirmed the proportion of 9.2% of IgM/IgG in current smokers, compared with 19.6% of non-smokers (former and never) [26]. Compared with this previous evidence and other analogous studies [26,27,28], this survey allowed investigating the smoking status and history resolving problems related to self-reporting and, thus, eliminating possible bias. Furthermore, the use of community-based data also avoided selection bias associated with the use of case series, which raised questions about the representativeness of cases compared with the general population. It is worth also noting that previous research relayed on the assessment of smoking in hospitalized subjects, with a major limitation due to the lack of appropriate controls [20]. Another similar weakness can be inferred from studies that enrolled patients with confirmed SARS-CoV-2 infection, leaving outside asymptomatic or pauci-symptomatic individuals. Indeed many studies included online surveys, which found that the burden of COVID-19-like symptoms and self-reported SARS-CoV-2 infection were significantly associated with smoking in syndromic surveillance data [24, 34, 46, 47, 54]—consistently with our findings—with a limitation on objective identifying and quantifying of attributable symptoms [55]. In confirmation of this, Clift also highlighted that heavy smoking (i.e., above 20 cigarettes per day) was associated with a reduced risk of SARS-CoV-2 infection when weighting by the probability of having received a SARS-CoV-2 test, likely prescribed for the occurrence of overlapping symptoms [24, 46].
In contrast, current smoking has been identified as a possible risk factor for progression of the disease, and was associated with higher risks of severe COVID-19 outcomes and death in large population-based researches [24, 25].
More in general, as previously discussed, the research about the effects of active smoking on both infection and disease is still controversial [19, 21], with some methodological limitations and pitfalls found in the literature so far available, including the assessment of smoking status and history among study populations, and systematic ascertainment biases and confounders in case-series on hospitalized smokers which might have led to inaccurate determining the overall smoking-attributable risk across the research [20, 33]. Additionally, some research on the response to COVID-19 vaccines highlighted a link between smoking and the humoral response to COVID-19 vaccines with effects on IgG titre and kinetics, with smoking accelerating the decline in vaccine-induced antibodies titre [56,57,58]. If a similar smoking-attributable effect occurs with antibodies induced by natural SARS-CoV-2 infection, then a much lower prevalence of IgG positivity is to be expected in smokers. Taken together, these findings call for further research about the effect of smoking on COVID-19 and immunological response to both infection and vaccines [56,57,58].
The mechanisms by which tobacco use decreases the risk of SARS-CoV-2 infection (and increases the risk of severe prognosis in COVID-19 patients) are not fully understood. Anti-inflammatory properties mediated by α7 nicotinic acetylcholine receptors and reduction in membrane angiotensin-converting enzyme 2 (ACE-2) expression in bronchial cells—which could play a role in SARS-CoV-2 pathology—have been proposed [20, 26, 28, 59,60,61]. Coronaviruses bind the ACE-2 host cell receptors through homotrimeric spike protein (i.e., S1 and S2 subunit) of their envelope, and, therefore, ACE-2 expression on bronchial tissue is a strong determinant for coronaviruses infectivity. Some studies captured a significant decrease of membrane ACE-2 protein expression attributable to cigarette smoking [61,62,63,64,65]. Again, this effect on ACE-2 might be likely attributable to acute smoking exposure, and thus unlikely to be associated with smoking duration, as revealed by our analyses.
Furthermore, Farsalinos et al. speculated that an anti-inflammatory pathway induced by nicotinic acetylcholine receptor might modulate the immune response from hyper-inflammation stimulated in severe COVID-19 [20, 66]. The cholinergic anti-inflammatory pathway, mediated mainly through the vagus nerve, represents a reflex mechanism based on a bi-directional communication between the immune and nervous systems [67, 68].
It can restore immune homeostasis and prevent cytokine storm, a hallmark of severe COVID-19. This hypothesis warrants further study, but the authors also suggested that the cessation of nicotine intake in hospitalized smokers leads to dysregulation of the cholinergic anti-inflammatory pathway and uncontrolled immune response, and was thus responsible for higher risk for severe outcomes [20]. Recently, a pharmaceutical company reported that α7 cholinergic agonists exhibit antiviral properties both in vitro and in vivo in experimental animals (macaques), but more clinical evidence is needed to verify or reject this hypothesis [69]. Smoking is a leading cause of morbidity and mortality worldwide, and smokers should be encouraged to quit for reducing the heavy burden associated with tobacco use [15, 58]. Obviously, even if results of a low infection rate among smokers will be confirmed in further study, smoking must not be perceived as a protective measure for COVID-19, neither encouraged nor recommended. However, the possibility for therapeutic effects of nicotine or nicotinic-cholinergic agonists on COVID-19 warrants further investigation by the research community through experimental in vitro studies and in clinical trials [58, 61, 70].
This paper has a number of strengths and weaknesses. The study is the first one to use objective measure of the smoking status, thus avoiding reporting bias and allowing to precisely detecting active smoking among participants. It also uses a specific and sensitive antibody assay, which accurately correlate with SARS-CoV-2 infection. Moreover, the field collection of the samples was conducted well before the launch of the national mass vaccination campaign, an important confounder in sero-epidemiologic studies. This is a unique aspect and constitutes a non-replicable added value of this research, as future studies will not be able to discount the possible confounding role of vaccine-induced IgG.
Lastly, the cohort was carefully assembled and sample size satisfactory, being representative of the population aimed to study and thus providing reliable estimates of the association between SARS-CoV-2 infection risk and smoking.
Despite these strengths, some limitations should be acknowledged. First, the limited number of hospitalized subjects did not allow inferring conclusions on this sub-group, leaving outside important aspects related to association of smoking and COVID-19 outcomes. Second, possible recall and notoriety biases should be acknowledged regarding the self-reported COVID-19 related symptoms, for this reason we excluded some possible confounders that could have affected the reliability of the data (e.g., duration of symptoms, etc.) [46, 55]. Third, our analysis was designed to investigate sero-positivity and no relationship between IgG titres and COVID-19 outcomes or smoking could be inferred.
In conclusion, this study documents a lower proportion of positive SARS-CoV-2 serology among current smokers, using direct laboratory measures of tobacco exposure and thus avoiding possible bias associated with self-reported smoking status. As such, the research captures actionable metric on the role of smoking in SARS-CoV-2 infection and COVID-19 outcomes, and contributes to refine current epidemiological risk estimates. Results may also serve as a reference for future clinical research on potential pharmaceutical role of nicotine or nicotinic-cholinergic agonists in COVID-19.
Data availability
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACE-2:
-
Angiotensin-converting enzyme 2
- COVID-19:
-
Coronavirus disease 2019
- ELISA:
-
Enzyme-linked immunosorbent assay
- HCW:
-
Healthcare workers
- IgG:
-
Immunoglobulin class G
- IRCCS:
-
Research Health Institute
- ISTAT:
-
Italian National Institute of Statistics
- PSM:
-
Propensity score matching
- SARS-CoV-2:
-
Severe acute respiratory syndrome—coronavirus 2
References
World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. Accessed 7 Feb 2022
Balasco N et al (2021) Analysis of the time evolution of COVID-19 lethality during the first epidemic wave in Italy. Acta Biomed 92(2):e2021171. https://doi.org/10.23750/abm.v92i2.11149
Ferrara P, Albano L (2020) COVID-19 and healthcare systems: what should we do next? Public Health 185:1–2. https://doi.org/10.1016/j.puhe.2020.05.014
Jamil S, Mark N, Carlos G et al (2020) Diagnosis and management of COVID-19 disease. Am J Respir Crit Care Med 201(10):P19–P20. https://doi.org/10.1164/rccm.2020C1
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Ponticelli D, Madotto F, Conti S et al (2021) Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: a 3-month follow-up. Intern Emerg Med. https://doi.org/10.1007/s11739-021-02857-y
Guan WJ, Liang WH, Zhao Y et al (2020) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547
Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN (2020) Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 382:e102
Caci G, Albini A, Malerba M et al (2020) COVID-19 and obesity: dangerous liaisons. J Clin Med 9(8):2511
Conti S, Ferrara P, Mazzaglia G et al (2020) Magnitude and time-course of excess mortality during COVID-19 outbreak: population-based empirical evidence from highly impacted provinces in northern Italy. ERJ Open Res 6(3):00458–02020. https://doi.org/10.1183/23120541.00458-2020
Harrison SL, Buckley BJR, Rivera-Caravaca JM et al (2021) Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes 7(4):330–339. https://doi.org/10.1093/ehjqcco/qcab029
Polosa R, Spinicci M, Prisco D (2020) “COVID-19: diagnosis, management and prognosis”: a new topical collection of Internal and Emergency Medicine. Intern Emerg Med 15(5):747–750. https://doi.org/10.1007/s11739-020-02461-6
World Health Organization Tobacco (2020). Newsroom. https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed 02 Feb 2021
Institute for Health Metrics and Evaluation Findings from the Global Burden of Disease Study 2017. Institute for Health Metrics and Evaluation. 2018. http://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf. Accessed 27 Jan 2021
Reitsma MB, Kendrick PJ, Ababneh E et al (2021) Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397(10292):2337–2360. https://doi.org/10.1016/S0140-6736(21)01169-7
US Department of Health and Human Services (2014) The health consequences of smoking: 50 years of progress: a report of the surgeon general. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta
Almirall J, Bolíbar I, Serra-Prat M et al (2008) Community-Acquired Pneumonia in Catalan Countries (PACAP) Study Group. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J 31(6):1274–1284. https://doi.org/10.1183/09031936.00095807
Lawrence H, Hunter A, Murray R, Lim WS, McKeever T (2019) Cigarette smoking and the occurrence of influenza—systematic review. J Infect 79(5):401–406. https://doi.org/10.1016/j.jinf.2019.08.014
Polosa R, Caci G (2020) COVID-19: counter-intuitive data on smoking prevalence and therapeutic implications for nicotine. Intern Emerg Med 15(5):853–856. https://doi.org/10.1007/s11739-020-02361-9
Farsalinos K, Bagos PG, Giannouchos T et al (2021) Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: an expanded re-analysis of a recent publication. Harm Reduct J 18:9
Li Volti G, Caruso M, Polosa R (2020) Smoking and SARS-CoV-2 disease (COVID-19): dangerous liaisons or confusing relationships? J Clin Med 9(5):1321. https://doi.org/10.3390/jcm9051321
Hou H, Li Y, Zhang P et al (2021) Smoking is independently associated with an increased risk for COVID-19 mortality: a systematic review and meta-analysis based on adjusted effect estimates. Nicotine Tob Res 23(11):1947–1951. https://doi.org/10.1093/ntr/ntab112
Patanavanich R, Glantz SA (2020) Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa082
Clift AK, von Ende A, Tan PS et al (2021) Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. https://doi.org/10.1136/thoraxjnl-2021-217080
Williamson EJ, Walker AJ, Bhaskaran K (2020) OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature 584:430–436. https://doi.org/10.1038/s41586-020-2521-4
Della Valle P, Fabbri M, Madotto F et al (2021) Occupational exposure in the lombardy region (Italy) to SARS-CoV-2 infection: results from the MUSTANG–OCCUPATION–COVID-19 study. Int J Environ Res Public Health 18(5):2567
Hippisley-Cox J, Young D, Coupland C et al (2020) Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 106:1503–1511. https://doi.org/10.1136/heartjnl-2020-317393
Israel A, Feldhamer I, Lahad A, Levin-Zamir D, Lavie G (2020) Smoking and the risk of COVID-19 in a large observational population study. medRxiv. https://doi.org/10.1101/2020.06.01.20118877
Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P, Vettor R (2020) Current smoking is not associated with COVID-19. Eur Respir J 55(6):2001290
Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto NR (2020) Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis 11:2040622320935765
Miyara M, Tubach F, Pourcher V et al (2020) Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. https://www.qeios.com/read/article/574. Accessed 15 Nov 2021
Farsalinos K, Barbouni A, Niaura R (2020) Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 9:1–8. https://doi.org/10.1007/s11739-020-02355-7
Polosa R, Tomaselli V, Ferrara P et al (2021) Seroepidemiological survey on the impact of smoking on SARS-CoV-2 infection and COVID-19 outcomes: protocol for the Troina study. JMIR Res Protoc 10(11):e32285. https://doi.org/10.2196/32285
Jackson SE, Brown J, Shahab L et al (2020) COVID-19, smoking and inequalities: a study of 53 002 adults in the UK. Tob Control. https://doi.org/10.1136/tobaccocontrol-2020-055933
Sakurai A, Sasaki T, Kato S et al (2020) Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med 383:885–886
National Center for Biotechnology Information. PubChem Compound Summary for CID 854019, Cotinine. https://pubchem.ncbi.nlm.nih.gov/compound/Cotinine. Accessed 1 Feb 2022
Istituto Superiore di Sanità. Sorveglianza integrata COVID-19. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati. Accessed 1 Feb 2022
World Health Organization (2020) Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection, 17 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331656. License: CC BY-NC-SA 3.0 IGO
Kim S (2016) Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health 13(12):1236. https://doi.org/10.3390/ijerph13121236
Ponticelli D, Antonazzo IC, Caci G et al (2021) Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J Travel Med. https://doi.org/10.1093/jtm/taab173
EUROIMMUN Anti-SARS-CoV-2 ELISA-EI 2606-9601 G-Product data sheet. EUROIMMUN Medizinische Labordiagnostika AG, Luebeck (Germany). https://www.coronavirus-diagnostics.com/antibody-detection-tests-for-covid-19.html. Accessed 15 Nov 2021
Beavis KG, Matushek SM, Abeleda APF et al (2020) Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol 129:104468. https://doi.org/10.1016/j.jcv.2020.104468
Rassen JA, Shelat AA, Myers J et al (2012) One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 21:69–80. https://doi.org/10.1002/pds.3263
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Sta 10(2):150–161. https://doi.org/10.1002/pst.433
Mehta CR, Patel N (1995) Exact logistic regression: theory and examples. Stat Med 14(19):2143–2160
McDonald S, Van den Wijngaard C, Wielders C et al (2021) Risk factors associated with the incidence of self-reported COVID-19-like illness: data from a web-based syndromic surveillance system in the Netherlands. Epidemiol Infect 149:E129. https://doi.org/10.1017/S0950268821001187
Istituto Nazionale di Statistica (ISTAT) Primi risultati dell’indagine di sieroprevalenza SARS-CoV-2. https://www.istat.it/it/files/2020/08/ReportPrimiRisultatiIndagineSiero.pdf. Accessed 1 Feb 2022
Vena A, Berruti M, Adessi A et al (2020) Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J Clin Med 9:2780. https://doi.org/10.3390/jcm9092780
Garcia-Basteiro AL, Moncunill G, Tortajada M et al (2020) Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 11:3500. https://doi.org/10.1038/s41467-020-17318-x
Richard A, Wisniak A, Perez-Saez J et al (2021) Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: a population-based study. Scand J Public Health. https://doi.org/10.1177/14034948211048050
Stock AD, Bader ER, Cezayirli P et al (2020) COVID-19 infection among healthcare workers: serological findings supporting routine testing. Front Med 7:471. https://doi.org/10.3389/fmed.2020.00471
Moscola J, Sembajwe G, Jarrett M et al (2020) Northwell Health COVID-19 research consortium prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City Area. JAMA 324:893–895. https://doi.org/10.1001/jama.2020.14765
Iversen K, Bundgaard H, Hasselbalch RB et al (2020) Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Inf Dis 20:P1401–P1408. https://doi.org/10.1016/S1473-3099(20)30589-2
Hopkinson NS, Rossi N, El-Sayed Moustafa J et al (2021) Current smoking and COVID-19 risk: results from a population symptom app in over 2.4 million people. Thorax. https://doi.org/10.1136/thoraxjnl-2020-216422
Amin-Chowdhury Z, Ladhani SN (2021) Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 27(7):1129–1130. https://doi.org/10.1038/s41591-021-01402-w
Ponticelli D, Antonazzo IC, Caci G et al (2021) Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J Travel Med 28(8):taab173. https://doi.org/10.1093/jtm/taab173
Ferrara P, Ponticelli D, Agüero F et al (2022) Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from VASCO study and need for further studies. Public Health 203:97–99. https://doi.org/10.1016/j.puhe.2021.12.013
Ferrara P, Gianfredi V, Tomaselli V, Polosa R (2022) The effect of smoking on humoral response to COVID-19 vaccines: a systematic review of epidemiological studies. Vaccines 10(2):303. https://doi.org/10.3390/vaccines10020303
Farsalinos K, Barbouni A, Niaura R (2020) Smoking, vaping and hospitalization for COVID-19. Qeios ID: Z69O8A.2. https://doi.org/10.32388/Z69O8A.2
Farsalinos K, Niaura R, Le Houezec J et al (2020) Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Version 2. Toxicol Rep 7:658–663. https://doi.org/10.1016/j.toxrep.2020.04.012
Caruso M, Distefano A, Emma R et al (2021) Role of cigarette smoke on angiotensin-converting enzyme-2 protein membrane expression in bronchial epithelial cells using an air–liquid interface model. Front Pharmacol 12:652102. https://doi.org/10.3389/fphar.2021.652102
Leun JM, Yang CX, Tam A et al (2020) ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 55(5):2000688. https://doi.org/10.1183/13993003.00688-2020
Leun JM, Yang CX, Sin DD (2020) COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J 55(6):2001261. https://doi.org/10.1183/13993003.01261-2020
Russo P, Bonassi S, Giacconi R et al (2020) COVID-19 and smoking: is nicotine the hidden link? Eur Respir J 55(6):2001116. https://doi.org/10.1183/13993003.01116-2020
Wrapp D, Wang N, Corbett KS et al (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483):1260–1263. https://doi.org/10.1126/science.abb2507
Conti P, Ronconi G, Caraffa A et al (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. https://doi.org/10.23812/CONTI-E
Wang H, Yu M, Ochani M et al (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388
Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859
Nau J, Luthra P, Lanzer K et al Varenicline prevents SARS-CoV-2 infection in vitro and in rhesus macaques. bioRxiv 2021.06.29.450426
Farsalinos K, Eliopoulos E, Leonidas DD, Papadopoulos GE, Tzartos S, Poulas K (2020) Nicotinic cholinergic system and COVID-19: in silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int J Mol Sci 21(16):5807
Acknowledgements
The authors would like to thank Concita Santoro, Annemarie Linder and Elisa Interlandi (CIDEC Federazione Sanità) for their invaluable assistance with collection and analysis of blood samples and to Fabio Venezia (the Mayor of Troina), Alessandro Nasca (Chief of Staff of Protezione Civile), and all the volunteers of Protezione Civile for their help with logistics. We are also grateful to Jas Mantero (Ending Pandemics) and Mark Smolinski (Ending Pandemics) for the fruitful scientific advice. Alessia Visalli (Euroimmun Italia Srl) for the technical discussion about specificity and sensibility of anti-SARS-CoV-2 IgG, and Adrian Shalivari (Fullcro Srl, Roma, Italy) for the development of the electronic data capture system. The paper is part of the research line on vulnerability and risk management of the project GRIDAVI — Risk Management, Decision Uncertainties and Social Vulnerabilities (Gestione del Rischio, Incertezze DecisionAli e Vulnerabilità sociali) by the University of Catania Research Incentive Plan 2020/2022 PIACERI. The authors' thoughts and prayers are with all the victims of the Russian-Ukrainian conflict.
Funding
This investigator-initiated research was sponsored by ECLAT srl, a spin-off of the University of Catania, with the help of a grant from the Foundation for a Smoke-Free World Inc., a US nonprofit 501(c)(3) private foundation with a mission to end smoking in this generation. The contents, selection, and presentation of facts, as well as any opinions expressed herein are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the Foundation for a Smoke-Free World, Inc. ECLAT srl. is a research-based company from the University of Catania that delivers solutions to global health problems with special emphasis on harm minimization and technological innovation.
Author information
Authors and Affiliations
Contributions
RP conceived the study. All authors contributed to design the study. VT and PF analysed data. VT, PF, and RP wrote the manuscript. VT and RP provided overall guidance and managed the overall project. All authors read and edited the manuscript. All authors approved the final version and the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
Outside this work, JR has received research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, JUUL Labs. Consulting with Revive pharmaceuticals, and consulting and patent purchase agreement with Philip Morris International. RP is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. In relation to his recent work in the area of respiratory diseases, clinical immunology, and tobacco control, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy no-profit organizations. RP has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network (IRIS) initiative. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., Health Diplomats, and Sermo Inc. RP has served on the Medical and Scientific Advisory Board of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc, Pfizer, and PharmaCielo. RP is also the founder of the Center for Tobacco prevention and treatment (CPCT) at the University of Catania and of the Center of Excellence for the acceleration of HArm Reduction (CoEHAR) at the same University, which has received support from Foundation for a Smoke-Free World to conduct eight independent investigator-initiated research projects on harm reduction. RP currently involved in a patent application concerning an app tracker for smoking behaviour developed for ECLAT Srl. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). All other authors have no conflict of interest to declare.
Ethics approval
The Research Ethics Committee of the IRCCS Oasi Maria Santissima (Troina, Enna, Italy) approved the research protocol, survey instrument, and informed consent form (approval n. 11/2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomaselli, V., Ferrara, P., Cantone, G.G. et al. The effect of laboratory-verified smoking on SARS-CoV-2 infection: results from the Troina sero-epidemiological survey. Intern Emerg Med 17, 1617–1630 (2022). https://doi.org/10.1007/s11739-022-02975-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-02975-1