Abstract
Either hypoglycemia or hypoalbuminemia alone is an independent condition associated with increased risk of mortality in critical illness. This study evaluates whether the mortality risk increases in septic patients if these conditions are combined. Patients admitted to our hospital from 2008 to 2015 who satisfied the definition of sepsis were targeted (n = 336). We classified cases into three groups based on blood glucose (BG) level measured at admission: hypoglycemia (Hypo-G; BG < 80 mg/dl), intermediate glycemia (Inter-G; 80–199 mg/dl), and hyperglycemia (Hyper-G; ≥ 200 mg/dl) group, and then estimated mortality. We also compared the clinical data of these glycemic groups in combination with hypoalbuminemia (Hypo-A) or Inter-G with non-hypoalbuminemia (Inter-G + Nonhypo-A), as a secondary analysis. Diagnostic cut-off level of Hypo-A (< 2.8 mg/dl) was determined using the ROC curve between blood albumin and mortality. In Hypo-G group (n = 40), APACHE II/SOFA scores are significantly higher than in the Inter-G (n = 196) and Hyper-G groups (n = 100). Mortality is 52.5% in the Hypo-G and 60.0% in the Hypo-G with Hypo-A (Hypo-G + Hypo-A) groups. Significantly higher APACHE II or SOFA scores and mortality are observed in the Hypo-G + Hypo-A group compared to the Inter-G + Nonhypo-A group. A higher mortality risk is observed in cases with Hypo-G + Hypo-A (OR 5.065) than those with Hypo-G (OR 3.503), Inter-G (OR 1.175), Hyper-G (OR 1.756) or Hypo-A (OR 3.243), calculated by a single logistic-regression analysis. Hypo-G + Hypo-A in patients with sepsis is related to higher ICU mortality. Physicians should be keenly aware of these conditions to provide immediate intensive treatment after admission of septic patients.
Similar content being viewed by others
Background
Hypoglycemia during hospitalization is associated with an increased hospital stay and contributes to a poorer outcome in diabetes patients in general medical wards in diabetic patients [1, 2]. While clinical evidence indicates that hypoglycemia following infectious illness in non-diabetic patients, such as pneumonia, is linked to poor outcomes [3, 4], hypoalbuminemia is also often observed in critically ill patients, and is related to outcome [5]. Recently, we report that low albumin is a significant predictor related to poor outcome in septic patients [6]. These studies have addressed either hypoglycemia or hypoalbuminemia alone as an independent risk factor that affects mortality in patients with critical illness. The significance of hypoglycemia with hypoalbuminemia in septic patients, however, has yet to be clarified. Early identification of high mortality risk in septic patients is an important clinical issue to improve overall outcome [7,8,9]. We hypothesize that hypoglycemia with hypoalbuminemic conditions may be related to an increased risk of mortality in septic patients. The objective of this study was to determine whether hypoglycemia with hypoalbuminemia at the time of admission might predict a higher risk of mortality in patients with sepsis.
Methods
This study was approved by the Clinical Research Review Committee of Nihon University School of Medicine (RK-160808-1) and was designed as a single-institution retrospective observational investigation using the database of patients treated for sepsis at our hospital. Sepsis is defined as infection plus systemic manifestations of infection [10], in patients admitted to the intensive care unit (ICU) of this hospital between January 2008 and December 2015.
In this study, data on the patients were obtained from the database system at our hospital. Every morning, team members (physicians, nurses, a pharmacologist, and an emergency medical technician) in the emergency department held a conference to review and discuss the details of the clinical record of each patient newly transferred to our department, including many factors related to outcome. All data for this study were obtained from the database and our patients’ clinical records.
Cases in which treatment for sepsis had already began in another hospital before admission to our hospital, cases of transfer after only resuscitation treatment at our hospital, and cases where details regarding pre-hospital events were incomplete, were excluded from this study.
To confirm infectious illness diagnosis, blood culture and whole body CT scans of the infection source were performed in this study.
Peripheral whole blood was collected from patients at admission. Patient information and laboratory data were recorded, including age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II score [11], and sequential organ failure assessment (SOFA) sore [12].
Cases were classified into three groups based on blood glucose level measured at admission: a hypoglycemia (hypo-G) group (blood glucose level < 80 mg/dl), a hyperglycemia (hyper-G) group (blood glucose level ≥ 200 mg/dl), and an intermediate glycemia (inter-G) group (blood glucose level 80–199 mg/dl) [13, 14].
Blood albumin levels were used to determine hypoalbuminemia associated with mortality according to receiver operating characteristic (ROC) curve-derived cut-off values. According to the ROC curve, the area under the curve (AUC) was 0.68 for the blood albumin (sensitivity 0.72, 1-specificity 0.43) and the value of blood albumin concentration was 2.8 mg/dl. In this study, a blood albumin level < 2.8 mg/dl is defined as hypoalbuminemia (Hypo-A).
As a secondary analysis, the effect of hypoalbuminemia in different combinations of hyperglycemia or hypoglycemia at the time of admission was estimated in septic patients. Glucose levels in patients with hypoalbuminemia at the time of admission were divided into separate groups and analyzed. Group distribution is as follows—Hypo-G + Hypo-A: Patients with a glycemic level < 80 mg/dl and blood albumin < 2.8 mg/dl, Inter-G + Hypo-A: patients with a glycemic level between 80 and 199 mg/dl and blood albumin < 2.8 mg/dl, and Hyper-G + Hypo-A: Patients with glycemic level ≥ 200 mg/dl and blood albumin < 2.8 mg/dl. As the control group, Inter-G + Nonhypo-A (non-hypoalbuminemia) was defined as patients with a glycemic level between 80 and 199 mg/dl and blood albumin over 2.8 mg/dl. In each Hypo-A, Inter-G + Nonhypo-A, Hypo-G + Hypo-A, Inter-G + Hypo-A and Hyper-G + Hypo-A group, there were some cases which were included in more than one group.
Outcome was evaluated at 28 ICU days or when the patient was discharged or transferred from our hospital.
Statistical analysis
All analyses were conducted using SPSS (IBM Statistics Version 22, Chicago, IL, USA) and JMP ver. 11.0 (SAS Institute, Cary, NC, USA). Data were presented as mean values [standard deviation (SD)] or number of cases (%). p < 0.05 was set as the definition of statistical significance.
Continuous variables were compared using Student’s t test or the Mann–Whitney U test as appropriate. The Pearson exact test was performed for categorical variables. Physiological data from each glycemic condition group were compared by one-way analysis of variance (ANOVA) or Kruskal–Wallis analysis. Subsequently, Tukey–Kramer or Steel–Dwass’s post hoc test was performed.
Outcome was predicted by multiple logistic-regression and calculating the odds ratios (OR) and 95% confidence interval (CI). Variables with p values < 0.2 by bivariate analysis were then introduced into the multivariate model [15]. The multivariate model was used to determine previously described clinical factors related to outcome as explanatory variables.
Multicollinearity, assessed using variance inflation factors [16], was detected among age, bilirubin, platelet, creatinine and APACHE II score or SOFA score, and among blood glucose levels (each glycemic group), and between blood albumin levels (cases with hypoalbuminemia); these variables were appropriately chosen in the multivariate model.
The Hypo-A, Inter-G + Nonhypo-A, Hypo-G + Hypo-A, Inter-G + Hypo-A and Hyper-G + Hypo-A groups were not included in the multivariate model because of multicollinearity due to some patients being in multiple groups. Finally, predicted outcome of patients in each glycemic condition was considered using a single regression analysis and the relationship between each glycemic condition group and hypoalbuminemia at admission was estimated.
Results
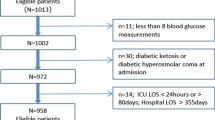
Five hundred and thirty consecutive cases of patients with sepsis were enrolled during the study period. After excluding 184 who had already begun the treatment at another hospital and 8 patients with incomplete data, 336 septic patients (204 males, 132 females) were included in this study.
The origins of the infection focus of the study patients were lung (pneumonitis; n = 176), urine tract (pyelonephritis; n = 53), abdomen (perforation of colon, n = 31), soft tissue (n = 19), central nervous system (n = 6), endocardial system (endocarditis; n = 2), and others, including unknown origin (n = 49) (Table 1).
Background and outcome of this study could be demonstrated when the patients were divided into two groups: a survival group and a non-survival group (Table 2). The significant higher values in APACHE II/SOFA score, higher GOT, higher BUN, lower blood albumin level, and metabolic acidosis were observed in the non-survival group although total bilirubin and blood glucose levels between groups are not significantly different (Table 2a). When the patients are divided into three groups based on blood glucose level measured at admission, the number of each group is as follows: 40 (11.9%) patients in the hypo-G group, 196 (58.3%) patients in the inter-G group, 100 (29.8%) patients in the hyper-G group. Distribution of all glycemic groups is shown in Table 2b. A significantly higher number of non-survivors is observed in the Hypo-G, Hyper-G, Hypo-A, Hypo-G + Hypo-A and Inter-G + Hypo-A groups, excluding the inter-G, Inter-G + Nonhypo-A and Hyper-G + Hypo-A groups. Conversely, Inter-G + Nonhypo-A group shows a significantly higher number of survivors compared to non-survivors.
Independent predictors of non-survivors are shown in Table 3. Multiple logistic regression analysis of the initial laboratory data at admission shows that low albumin (OR 0.4617; 95% CI 0.2769–0.7549, p = 0.0019), lactate (OR 1.0703; 95% CI 1.0074–1.1416, p = 0.0027) or, the presence of hypoglycemia (Hypo-G; OR 3.1424; 95% CI 1.5649–6.3638, p = 0.0014) is associated with higher mortality. Other independent predictors of high mortality risk do not demonstrate a correlation.
Distribution of all glycemic groups for patients with and without hypoalbuminemia is shown in Table 4. All groups are compared with the Inter-G + Nonhypo-A group (control). Some cases were included in more than one group. APACHE II scores are significantly higher in the Hypo-G, Hyper-G, Hypo-A, and Hypo-G + Hypo-A groups compared to the control group, but SOFA scores are significantly higher than those of the Hypo-G and Hypo-G + Hypo-A groups, found using a multiple comparison test. Mortality is 52.5% in the Hypo-G, 40.2% in the Hypo-A, and 60.0% in the Hypo-G + Hypo-A group. Hypo-G + Hypo-A group shows a significantly higher rate (p < 0.0001) than the control group (15.2% in the Inter-G + Nonhypo-A group) using the Pearson exact test.
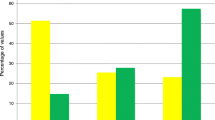
Figure 1 shows APACHE II scores (Fig. 1a), SOFA scores (Fig. 1b), and mortality (Fig. 1c) among each glycemic group and patients with or and without hypoalbuminemia. In particular, significantly higher scores and mortality are observed in the Hypo-G (p < 0.0001) and Hypo-G + Hypo-A (p < 0.0001) groups when classification criteria were met (black bars), compared to cases whose classification was not met (white bars).
APACHE II scores, SOFA scores and mortality for each glycemic group and with or without hypoalbuminemia. a APACHE II scores. b SOFA scores. c Mortality. Significant differences in APACHE II scores, SOFA scores and mortality are observed between In the Hypo-G and Hypo-G + Hypo-A groups and the other groups in cases with hypoalbuminemia (Positive: black bars), compared to cases without hypoalbuminemia (No: white bars in figure). APACHE II score and SOFA score were calculated using initial data from the emergency department. APACHE II score Acute Physiology and Chronic Health Evaluation score II, SOFA score sequential Organ Failure Assessment score, Negative patients who did not meet the classification criteria for the specified group are indicated by white bars, Positive patients who met the classification criteria for the specified group are indicated by black bars, Hypo-G hypoglycemia group (blood glucose level < 80 mg/dl), Hyper-G hyperglycemia group (blood glucose level ≥ 200 mg/dl), Inter-G intermediate glycemia group (blood glucose level = 80–199 mg/dl), Hypo-A hypoalbuminemia (blood albumin < 2.8 mg/dl), Nonhypo-A non-hypoalbuminemia (blood albumin ≥ 2.8 mg/dl or more), Inter-G + Nonhypo-A patients with a glycemic level between 80 and 199 mg/dl and blood albumin 2.8 mg/dl or more, Hypo-G + Hypo-A patients with a glycemic level < 80 mg/dl and blood albumin < 2.8 mg/dl, Inter-G + Hypo-A patients with a glycemic level between 80 and 199 mg/dl and blood albumin < 2.8 mg/dl, Hyper-G + Hypo-A patients with a glycemic level ≥ 200 mg/dl and blood albumin < 2.8 mg/dl. Some cases in each Inter-G + Norhypo-A, Hypo-G + Hypo-A, Inter-G + Hypo-A and Hyper-G + Hypo-A group were included in more than one group
APACHE II or SOFA scores were used to determine whether the Hypo-G + Hypo-A group was associated with mortality, according to ROC curve-derived cut-off values. According to the ROC curve of the APACHE II or SOFA scores in the Hypo-G + Hypo-A group, AUC is 0.76 for the APACHE II score (sensitivity 0.75, 1-specificity 0.42), for the APACHE II score value of 26, and AUC is 0.78 for the SOFA score (sensitivity 0.71, 1-specificity 0.42) for the SOFA score value of 9.
Finally, a single logistic regression analysis for the initial laboratory data at emergency department admission shows that APACHE II score (OR 1.116; 95% CI 1.0753–1.1611, p < 0.0001), SOFA score (OR 1.256; 95% CI 1.1559–1.3737, p < 0.0001), Hypo-G (OR 3.503; 95% CI 1.7820–6.9351, p = 0.0003), Hypo-A (OR 3.243; 95% CI 1.9774–5.3957, p < 0.0001) or Hypo-G + Hypo-A (OR 5.065; 95% CI 2.1664–12.498, p = 0.0002), existing in each septic patient, is associated with higher mortality (Table 5).
Discussion
Due to high morbidity or mortality during intensive care, prompt clinical evaluation of severity in sepsis is an important strategy to improve outcome [17, 18]. To evaluate severity and mortality in critical illness, APACHE II score and SOFA score are generally used in the ICU. This study indicates a higher OR for mortality risk in patients with hypoglycemia (OR 3.503), hypoalbuminemia (OR 3.243), or both hypoglycemia and hypoalbuminemia (OR 5.065), than that estimated by SOFA score (OR 1.256). These results may be useful for early and easy identification of high-mortality risk in patients with sepsis at the time of hospital admission.
During systemic excess stress insults, blood glucose level usually increases by insulin resistance or secretion of adrenal hormones such as catecholamine [19,20,21]. These increased levels of glucose, or stress-induced hyperglycemia, is a normal and important physiologic response to stress that is common in critically ill patients [22, 23]. Hypoglycemia, however, has been considered a critical pathophysiological condition in pneumonia [3, 4] and in critically ill patients [24, 25]. There are reports that hypoglycemia is also an available prognosticator in patients with sepsis [26,27,28]. Previous reports, together with our results, demonstrate the hypoglycemia observed in the initial data at the hospital admission may be useful in indicating severity and in the prognostic prediction in septic patients. Hypoalbuminemia is in itself an effective and powerful indicator of mortality and morbidity in cases of sepsis [5]. Moreover, hypoglycemia under hypoalbuminemia conditions in septic patients should be considered as an alert sign for clinicians to provide early management.
The pathophysiology of hypoglycemia or hypoalbuminemia may have many mechanisms. In the experimental model of sepsis, glucose utilization would increase in macrophage-rich tissues, such as the liver, and then lead to hypoglycemia [29, 30] and decreased hepatic glucose production [30, 31]. Reduction in albumin synthesis by inflammatory reactions, such as many mediators, is well known [32, 33]. Vascular permeability increases during sepsis, leading to the transcapillary loss of albumin and acceleration to hypoalbuminemia [33,34,35]. Taken together, these patients may experience depression with hepatic gluconeogenesis, as a result of decreased sensitivity to stress hormones or adrenal failure [30] leading to hypoglycemia, and concomitant with reduced albumin synthesis and transcapillary loss caused by inflammatory reactions, depending on the severity of inflammation.
Although age or cardiac function is also an incremental prognostic value [36, 37], for early evaluation of sepsis, quick SOFA score and screening for patients suspected of having sepsis can be expected to start treatment earlier [38]. If the severity of sepsis and outcomes could be predicted using the initial simple data at admission after evaluation of sepsis using quick SOFA, physicians could provide a therapeutic plan earlier, in combination with various additional treatments for patients in the high-mortality group.
There are some limitations to this study. A major limitation is that this is a retrospective observational study of a limited number of patients in a single institution, so detailed information of patient nutritional conditions, such as body mass index and duration of illness before hospitalization, was not evaluated. This study also did not consider history of diabetes or the effect of diabetic agents since some patients had not received a medical check for a long time, and other patients had been diagnosed diabetes but did not take their medication. In the emergency department, glycemic value evaluation is greatly influenced by nutritional intake and antidiabetic therapy. Rather than acute onset, septic conditions continued for days and gradually deteriorated. Hence, accurate data regarding nutritional intake or antidiabetic medicine in cases of patients with diabetic history could not be obtained. Generally, estimating illness duration is difficult since many septic patients do not know the time of onset. Due to these reasons, this study could only identify patients who had a long duration of illness as reflected in collapsed pathophysiological conditions after systemic depletion due to infection. In addition, although hyperglycemia in septic patients at admission is well known to carry a high mortality risk, diabetes is not [22, 39, 40]. In this study, bias in initial blood glucose or albumin levels was present. Actually, data were obtained from patients only at the time of admission. The underlying mechanisms regarding how hypoglycemia in septic patients under hypoalbuminemia conditions is related to outcome still remain unclear. Finally some cases are included in more than one group. For multicollinearity reasons, multiple regression analysis using a multivariate model could not be performed for outcome evaluation. The odds ratio of mortality was calculated by single logistic regression analysis of each group.
Conclusion
Hypoglycemia with hypoalbuminemia at admission is related to higher ICU mortality in septic patients, compared with other glycemic conditions. A combination of simple laboratory data from the emergency may be useful to predict the severity and mortality after diagnosis of sepsis. Physicians should be keenly aware of these conditions to provide immediate intensive treatment after diagnosis of sepsis. Hypoglycemia with hypoalbuminemia may contribute to the underlying mechanisms in septic patients.
Change history
26 October 2019
In the original article, there were errors in Unit of serum albumin level and the Cases numbers in Table 4.
References
Tan HK, Flanagan D (2013) The impact of hypoglycaemia on patients admitted to hospital with medical emergencies. Diabet Med 30:574–580
Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML (2009) Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 32:1153–1157
Mortensen EM, Garcia S, Leykum L, Nakashima B, Restrepo MI, Anzueto A (2010) Association of hypoglycemia with mortality for subjects hospitalized with pneumonia. Am J Med Sci 339:239–243
Singanayagam A, Chalmers JD, Hill AT (2009) Admission hypoglycaemia is associated with adverse outcome in community acquired pneumonia. Eur Respir J 34:932–939
Gatta A, Verardo A, Bolognesi M (2012) Hypoalbuminemia. Intern Emerg Med 7(Suppl 3):S193–S199
Yamaguchi J, Kinoshita K, Ihara S, Furukawa M, Sakurai A (2018) The clinical significance of low serum arachidonic acid in sepsis patients with hypoalbuminemia. Intern Med 57:1833–1840
Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M (2010) Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 38:1045–1053
Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE (2006) The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis 193:251–258
Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, Kline JA, Emergency Medicine Shock Research Network investigators (2008) The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 36:2734–2739
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Seino Y, Nanjo K, Takashi Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K (2010) Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Diabetol Int 1:2–20
Moldonado G, Greenland S (1993) Simulation study of confounder selection strategies. Am J Epidemiol 138:923–936
Glantz SA, Slinker BK (1990) Primer of applied regression and analysis of variance. McGraw-Hill, New York, pp 181–199
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC (2016) Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:762–774
Vermes I, Beishuizen A (2001) The hypothalamic–pituitary–adrenal response to critical illness. Best Pract Res Clin Endocrinol Metab 15(4):495–511
Santos S (2013) Stress response in critical illness. Curr Probl Pediatr Adolesc Health Care 43:264–272
Marik PE, Raghavan M (2004) Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 30:748–756
Stegenga ME, Vincent JL, Vail GM, Xie J, Haney DJ, Williams MD, Bernard GR, van der Poll T (2010) Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med 38:539–545
Leverve X (2003) Hyperglycemia and oxidative stress: complex relationships with attractive prospects. Intensive Care Med 29:511–514
Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C, ANZICS CORE Management Committee (2009) The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care 13:R91
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M (2010) Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 85:217–224
Berkman P, Merdler C, Yust I (1985) Hypoglycemia as a manifestation of sepsis in an elderly patient. J Am Geriatr Soc 33:644–645
Bellomo R, Egi M (2011) Hypoglycemia in sepsis: biomarker, mediator, or both? Crit Care Med 39:2367–2369
Ssekitoleko R, Jacob ST, Banura P, Pinkerton R, Meya DB, Reynolds SJ, Kenya-Mugisha N, Mayanja-Kizza H, Muhindo R, Bhagani S, Scheld WM, Moore CC (2011) Hypoglycemia at admission is associated with inhospital mortality in Ugandan patients with severe sepsis. Crit Care Med 39:2271–2276
Lang CH, Dobrescu C (1991) Sepsis-induced increases in glucose uptake by macrophage-rich tissues persist during hypoglycemia. Metabolism 40:585–593
Maitra SR, Wojnar MM, Lang CH (2000) Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock 13:379–385
Clemens MG, Chaudry IH, McDermott PH, Baue AE (1983) Regulation of glucose production from lactate in experimental sepsis. Am J Physiol 244:R794–R800
Herrmann FR, Saqfran C, Levkoff SE, Minaker KL (1992) Serum albumin level on admission as a predictor of death, length of stay and readmission. Arch Intern Med 152:125–130
Nicholson JP, Wolmarans MR, Park GR (2000) The role of albumin in critical illness. Br J Anaesth 85:599–610
Ballmer PE (2001) Causes and mechanisms of hypoalbuminaemia. Clin Nutr 20:271–273
Ballmer PE, Ochsenbein AF, Schutz-Hofmann S (1994) Transcapillary escape rate of albumin positively correlates with plasma albumin concentration in acute but not in chronic inflammatory disease. Metabolism 43:697–705
Innocenti F, Tozzi C, Donnini C, De Villa E, Conti A, Zanobetti M, Pini R (2018) SOFA score in septic patients: incremental prognostic value over age, comorbidities, and parameters of sepsis severity. Intern Emerg Med 13:405–412
Innocenti F, Palmieri V, Guzzo A, Stefanone VT, Donnini C, Pini R (2018) SOFA score and left ventricular systolic function as predictors of short-term outcome in patients with sepsis. Intern Emerg Med 13:51–58
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
Schuetz P, Jones AE, Howell MD, Trzeciak S, Ngo L, Younger JG, Aird W, Shapiro NI (2011) Diabetes is not associated with increased mortality in patients with sepsis. Ann Emerg Med 58:438–444
van Vught LA, Holman R, de Jonge E, de Keizer NF, van der Poll T (2017) Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Crit Care Med 45:e1026–e1035
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and/or animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Since this is a retrospective study, patient consent was not obtained. This study was approved by the Clinical Research Review Committee of Nihon University School of Medicine (RK-160808-1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Furukawa, M., Kinoshita, K., Yamaguchi, J. et al. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med 14, 539–548 (2019). https://doi.org/10.1007/s11739-019-02034-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-019-02034-2