Abstract
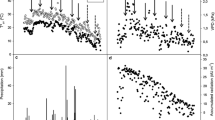
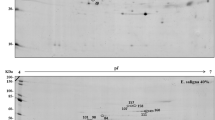
To understand the mechanisms of heat stress responses in perennial grasses, differential proteins in leaves and roots of two genotypes of Kentucky bluegrass (Poa pratensis), including heat-tolerant ‘Midnight’ and heat-sensitive ‘Brilliant’, were analyzed with two-dimensional gel electrophoresis (2-DE) and mass spectrometry (MS). Plants were exposed to heat stress for 28 days in growth chambers. Under 7–28 days of heat stress, leaf photochemical efficiency declined significantly while electrolyte leakage increased in leaves and roots, and to a lesser extent for heat-tolerant ‘Midnight’ than for heat-sensitive ‘Brilliant’. Compared with leaves, cell membrane damage due to heat stress was more severe in roots. The 2-DE and MS analysis identified 37 heat-responsive proteins in leaves, 28 heat-responsive proteins in roots; 14 proteins in leaves and 9 proteins in roots exhibited differential expression between the two genotypes. The results indicate that proteins involved in metabolism and energy in leaves and those in antioxidant defense in roots are associated with heat tolerance in Kentucky bluegrass. The differential accumulation of these proteins might be the reason for different heat tolerance in two Kentucky bluegrass genotypes in aerial and underground parts.










Similar content being viewed by others
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- MS:
-
Mass spectrometry
- Hsp:
-
Heat-shock proteins
- EL:
-
Electrolyte leakage
- Fv/Fm:
-
The ratio of variable to maximum fluorescence
- DTT:
-
Dithiothreitol
- CHAPS:
-
3-[(3-Cholamidopropyl) dimethylammoniol]-1-propane-sulfonate
- IPG:
-
Immobilized pH gradient
- IEF:
-
Isoelectric focusing
- MALDI-TOF/TOF:
-
Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight
- ESI-MS:
-
Electrospray ionization mass spectrometry
- CS:
-
Cysteine synthase
- TCA:
-
Tri-carboxylic-acid
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Cyt 450:
-
Cytochrome P450
- POD:
-
Peroxidase
- DHAR:
-
Dehydroascorbate reductase
- TPI:
-
Triosephosphate isomerase
- 3-PGK:
-
3-Phosphoglycerate kinase
- GSH:
-
Glutathione
- LGL:
-
Lactoylglutathionelyase
- MG:
-
Methylglyoxal
- ADK:
-
Adenosine kinase
- ROS:
-
Reactive oxygen species
- GLPs:
-
Germin-like proteins
- NAC:
-
Nascent polypeptide-associated complex
- P:
-
Phosphorus
References
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Ahsan N, Komatsu S (2009) Comparative analyses of the proteomes of leaves and flowers at various stages of development reveal organ-specific functional differentiation of proteins in soybean. Proteomics 9:4889–4907
Ahsan N, Lee DG, Lee SH, Kang KY, Bahk JD, Choi MS, Lee IJ, Renaut J, Lee BH (2007) A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiol Plant 131:555–570
Ahsan N, Donnart T, Nouri MZ, Komatsu S (2010) Tissue-specific defense and thermo-adaptive mechanisms of soybean seedlings under heat stress revealed by proteomic approach. J Proteome Res 9:4189–4204
Bayer EM, Bottrill AR, Walshaw J, Vigouroux M, Naldrett MJ, Thomas CL, Maule AJ (2006) Arabidopsis cell wall proteome defined using multidimensional protein identification technology. Proteomics 6:301–311
Becker TW, Carrayol E, Hirel B (2000) Glutamine synthetase and glutamate dehydrogenase isoforms in maize leaves: localization, relative proportion and their role in ammonium assimilation or nitrogen transport. Planta 211:800–806
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124:128–145
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol Plant Mol Biol 31:491–543
Bevan M, Bancroft I, Bent E, Love K, Goodman H, Dean C, Bergkamp R, Dirkse W, Van Staveren M, Stiekema W (1998) Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391:485–488
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Chiou TJ, Liu H, Harrison MJ (2001) The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J 25:281–293
DeLucia E, Callaway R, Thomas E, Schlesinger W (1997) Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Ann Bot 79:111–120
Ding SJ, Li Y, Tan YX, Jiang MR, Tian B, Liu YK, Shao XX, Ye SL, Wu JR, Zeng R, Wang HY, Tang ZY, Xia QC (2004) From proteomic analysis to clinical significance- overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis. Mol Cell Proteom 3:73–81
Du H, Ding M, Tang D, Huang D (2011) Analysis of differential protein expression during the early stage of in vitro tuberization in taro. Sci Hortic 129:904–909
Du H, Zhou P, Huang B (2013) Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ Exp Bot 87:159–166
Dunwell JM, Gibbings JG, Mahmood T, Saqlan Naqvi SM (2008) Germin and germin-like proteins: evolution, structure, and function. Crit Rev Plant Sci 27:342–375
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Ferreira S, Hjernø K, Larsen M, Wingsle G, Larsen P, Fey S, Roepstorff P, Pais MS (2006) Proteome profiling of Populus euphratica Oliv. upon heat stress. Ann Bot 98:361–377
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Hajheidari M, Abdollahian-Noghabi M, Askari H, Heidari M, Sadeghian SY, Ober ES, Hosseini Salekdeh G (2005) Proteome analysis of sugar beet leaves under drought stress. Proteomics 5:950–960
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agr Exp Stat 347:1–32
Jiang Y, Yang B, Harris NS, Deyholos MK (2007) Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot 58:3591–3607
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J Proteom 74:1301–1322
Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH (2007) A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics 7:3369–3383
Liu Y, Du H, He X, Huang B, Wang Z (2012) Identification of differentially expressed salt-responsive proteins in roots of two perennial grass species contrasting in salinity tolerance. J Plant Physiol 169:117–126
Marcum KB (1998) Cell membrane thermostability and whole-plant heat tolerance of Kentucky bluegrass. Crop Sci 38:1214–1218
Mendoza-Cózatl D, Loza-Tavera H, Hernández-Navarro A, Moreno-Sánchez R (2005) Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev 29:653–671
Miernyk JA (1999) Protein folding in the plant cell. Plant Physiol 121:695–703
Moffatt BA, Stevens YY, Allen MS, Snider JD, Pereira LA, Todorova MI, Summers PS, Weretilnyk EA, Martin-McCaffrey L, Wagner C (2002) Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol 128:812–821
Neuhoff V, Stamm R, Eibl H (1985) Clear background and highly sensitive protein staining with coomassie blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 6:427–448
Nieto-Sotelo J, Martinez LM, Ponce G, Cassab GI, Alagon A, Meeley RB, Ribaut JM, Yang RY (2002) Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 14:1621–1633
Oshino T, Abiko M, Saito R, Ichiishi E, Endo M, Kawagishi-Kobayashi M, Higashitani A (2007) Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high-temperature injury in barley plants. Mol Genet Genom 278:31–42
Perotti VE, Moreno AS, Trípodi KEJ, MeierG Bello F, Cocco M, Vázquez D, Anderson C, Podestá FE (2015) Proteomic and metabolomic profiling of Valencia orange fruit after natural frost exposure. Physiol Plant 153:337–354
Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47:185–214
Portis AR Jr (2003) Rubisco activase-Rubisco’s catalytic chaperone. Photosynth Res 75:11–27
Rachmilevitch S, Lambers H, Huang B (2006) Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. J Exp Bot 57:623–631
Rasoulnia A, Bihamta MR, Peyghambari SA, Alizadeh H, Rahnama A (2011) Proteomic response of barley leaves to salinity. Mol Biol Rep 38:5055–5063
Rospert S, Dubaquie Y, Gautschi M (2002) Nascent-polypeptide-associated complex. Cell Mol Life Sci 59:1632–1639
Shen H, Du H, Wang Z, Huang B (2009) Differential responses of nutrients to heat stress in warm-season and cool-season turfgrasses. HortScience 44:2009–2014
Sperisen C, Ryals J, Meins F (1991) Comparison of cloned genes provides evidence for intergenomic exchange of DNA in the evolution of a tobacco glucan endo-1,3-beta-glucosidase gene family. Proc Natl Acad Sci USA 88:1820–1824
Szalai G, Kellős T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Takahashi H, Saito K (1996) Subcellular localization of spinach cysteine synthase isoforms and regulation of their gene expression by nitrogen and sulfur. Plant Physiol 112:273–280
Thornalley P (2003) Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31:1343–1348
Turgeon AJ (2008) Turfgrass management, 8th edn. Pearson Prentice Hall, New Jersey
Turóczy Z, Kis P, Török K, Cserháti M, Lendvai Á, Dudits D, Horváth G (2011) Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol Biol 75:399–412
Udompraset N, Li PH, Davis DW, Markhart AH III (1995) Root cytokinin level in relation to heat tolerance of Phaseolus acutifolius and Phaseolus vulgaris. Crop Sci 35:486–490
Valdés-López O, Batek J, Gomez-Hernandez N, Nguyen CT, Isidra-Arellano MC, Zhang N, Joshi T, Xu D, Hixson KK, Weitz KK, Aldrich JT, Paša-Tolić L, Stacey G (2016) Soybean roots grown under heat stress show global changes in their transcriptional and proteomic profiles. Front Plant Sci 7:517. doi:10.3389/fpls.2016.00517
Vo K, Kruse S, Reski R, Moffatt B, Laloue M (1998) Cloning and characterization of an adenosine kinase from Physcomitrella involved in cytokinin metabolism. Plant J13:249–257
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Witzel K, Weidner A, Surabhi GK, Börner A, Mock HP (2009) Salt stress-induced alterations in the root proteome of barley genotypes with contrasting response towards salinity. J Exp Bot 60:3545–3557
Woo EJ, Dunwell JM, Goodenough PW, Marvier AC, Pickersgill RW (2000) Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat Struct Mol Biol 7:1036–1040
Xiao K, Liu J, Dewbre G, Harrison M, Wang ZY (2006) Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula. Plant Biol 8(4):439–449
Xu C, Huang B (2008) Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. J Exp Bot 59:4183–4194
Xu Y, Gianfagna T, Huang B (2010) Proteomic changes associated with expression of a gene (ipt) controlling cytokinin synthesis for improving heat tolerance in a perennial grass species. J Exp Bot 61:3273–3289
Xu Y, Zhang C, Huang B (2011) Heat shock proteins in association with heat tolerance in grasses. Int J Proteom. doi:10.1155/2011/529648
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11:1–11
Yan S, Tang Z, Su W, Sun W (2005) Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics 5:235–244
Yan S, Zhang Q, Tang Z, Su W, Sun W (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteom 5:484–496
Yano H, Kuroda M (2006) Disulfide proteome yields a detailed understanding of redox regulations: a model study of thioredoxin-linked reactions in seed germination. Proteomics 6:294–300
Yun Z, Jin S, Ding Y, Wang Z, Gao H, Pan Z, Xu J, Cheng Y, Deng X (2012) Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage. J Exp Bot 63:2873–2893
Zhang Y, Xu L, Zhu X, Gong Y, Xiang F, Sun X, Liu L (2013) Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.). Plant Mol Biol Rep 31:195–203
Zhao Y, Du H, Wang Z, Huang B (2011) Identification of proteins associated with water-deficit tolerance in C4 perennial grass species, Cynodon dactylon × Cynodon transvaalensis and Cynodon dactylon. Physiol Plant 141:40–55
Zou J, Liu C, Chen X (2011) Proteomics of rice in response to heat stress and advances in genetic engineering for heat tolerance in rice. Plant Cell Rep 30:2155–2165
Acknowledgments
The authors wish to thank National Natural Science Foundation of China (Grant No. 30900992) for funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Communicated by H. Peng.
Rights and permissions
About this article
Cite this article
Zhang, Y., Du, H. Differential accumulation of proteins in leaves and roots associated with heat tolerance in two Kentucky bluegrass genotypes differing in heat tolerance. Acta Physiol Plant 38, 213 (2016). https://doi.org/10.1007/s11738-016-2232-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2232-5