Abstract
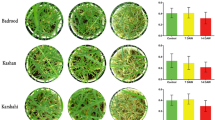
Festuca arundinacea Schreb. (tall fescue) is a turfgrass desired for planting in transition-zones. To determine the major processes associated with drought tolerance in this crop, four cultivars were analyzed for enzymatic and non-enzymatic antioxidant activity as well as SGR transcript level under drought stress. These cultivars were selected from larger set of eleven tall fescue cultivars based on genetic relationships delineated via inter-simple sequence repeat (ISSR) analysis. This analysis divided the cultivars into 6 groups, according to Jaccard’s similarity coefficient (65 %), and identified Jaguar, h–d, Pixie, and Mini-mustang as representing diversity within the samples. These cultivars were thus subjected to drought stress treatment by withholding irrigation for up to 8 days. By the end of the treatment, two of the four cultivars had decreased chlorophyll content; Jaguar and h–d, moderately drought-tolerant cultivars did not display significant differences. Notably, the FaSGR transcript increased drastically over the course of the drought stress in Pixie and Mini-Mustang, in contrast to Jaguar and h–d, supporting the notion of SGR-mediated chlorophyll degradation in the less drought-tolerant cultivars. Biochemical adaptation to drought stress includes accumulation of solutes such as proline to maintain turgor, and detoxification of drought-associated reactive oxygen species. Pixie, Jaguar, and Mini-mustang exhibited drastic changes in proline content as compared to h–d, possibly due to their dependency on proline in order to maintain cellular homeostasis against drought stress. The drought-tolerant h–d may accumulate only slightly more proline due to other drought-tolerance adaptations. Water scarcity during drought stress significantly increased superoxide dismutase (SOD) and ascorbate peroxidase (APX) activity in all cultivars, though the extent of induction was correlated with drought susceptibility. This suggests that most of the superoxide that was produced as a result of drought stress was converted to H2O2 by SOD and subsequently detoxified by APX into H2O. Collectively, these data indicate that the cultivars Pixie and Mini-mustang protect critical cellular enzymes and structures during severe drought stress through drastically increased proline and SOD activity, while the more drought-tolerant h–d and jaguar do not require such extreme biochemical modifications.








Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide scavenging enzyme in plants. Physiol Plant 85:235–241
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Plant Mol Biol 50:601–639
Bates LS, Waldern RP, Teare ID (1973) Rapid determination of free proline for water stress study. Plant Soil 39:205–207
Ben Ghnaya A, Charles G, Hourmant A, Ben Hamida J, Branchard M (2007) Morphological and physiological characteristics of rapeseed plants regenerated in vitro from thin layers in the presence of zinc. Plant Biol Pathol 330:728–734
Carrow RN (1996) Drought avoidance characteristics of diverse tall fescue cultivars. Crop Sci 36:371–377
Chance B, Maehly A (1995) Assay of catalase and peroxidase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 764–775
Cross JW, Bonos SA, Huang B, Meyer WA (2013) Evaluation of heat and drought as components of summer stress on tall fescue genotypes. HortScience 48:1562–1567
Cui L, Li J, Fan Y, Xu S, Zhang Z (2006) High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Botanical Studies 47:61–69
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Doyle JJ, Doyle JL (1990) Isolation of plaant DNA from fresh tissue Focus 12:13–15
Esmaili S, Salehi H (2012) Effects of temperature and photoperiod on postponing bermudagrass (Cynodon dactylon [L.] Pers.) turf dormancy. J Plant Physiol 169:851–858
Feierabend J, Engel S (1986) Photoinactivation of catalase in vitro and in leaves. Arch Biochem Biophys 251:567–576
Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxid Redox Signal 18:2087–2090
Foyer CH, Descourvierse P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Huang B, DaCosta M, Jiang Y (2014) Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Crit Rev Plant Sci 33:141–189
Ji Y, Zhang X, Peng Y, Huang L, Liang X, Wang K, Yin G, Zhao X (2014) Osmolyte accumulation, antioxidant enzyme activities and gene expression patterns in leaves of orchardgrass during drought stress and recovery. Grass Sci 60:1361–1362
Jiang M, Zhang J (2002) Involvement of plasma-membrane NADPH oxidase in abscisic acid and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Kosmala A, Perlikowski D, Pawlowicz Rapacz M (2012) Changes in the chloroplast proteome following water deficitand subsequent watering in a high- and a low-drought-tolerant genotype of Festuca arundinacea. J Exp Bot 63:6161–6172
Kumar V, Shriram V, Kavi Kishor PB, Jawali M, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep 4:37–48
Larionov A, Krause A, Miller W (2005) A standard curve based method for relative qRT-PCR data processing. BMC Bioinformatics 6:62. doi:10.1186/1471-2105-6-62
Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Liu J, Xie X, Du J, Sun J, Bai J (2008) Effects of simultaneous drought and heat stress on Kentucky bluegrass. Sci Hort 115:190–195
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S (2012) H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem 287:11717–11729
Matysik J, Bhalu AB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxide in spinach chloroplast. Plant Cell Physiol 22:867–880
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165
Perlikowski D, Kosmala A, Rapacz M, Koscielniak J, Pawlowicz Zwierzykowski Z (2013) Influence of short-term drought conditions and subsequent re-watering on the physiology and proteome of Lolium multiflorum/Festuca arundinacea introgression forms, with contrasting levels of tolerance to long-term drought. Plant Biology 16:385–394
Polle A (1997) Defense against photooxidative damage in plants [A]. oxidative stress and the molecular biology of antioxidant defense [C] Harbor, Cold Spring Harbor Laboratory Press, Cold Spring, NY, pp 785–813
Richards LA (1949) Methods of measuring soil moisture tension. Soil Sci 68:95–112
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Sairam RK, Srivastava GC, Saxena DC (2000) Increased antioxidant activity under elevated temperatures, a mechanism of heat stress tolerance in wheat genotypes. Biol Plant 43:245–251
Sakuraba Y, Dami K, Kim YS, Hortensteiner S, Paek NC (2014a) Arabidopsis STAYGREEN-LIKE (SGRL) promotes abiotic stress-induced leaf yellowing during vegetative growth. FEBS Lett 588:3830–3837
Sakuraba Y, Parka SY, Kima YS, Wang SH, Yood SH, Hortensteiner S, Paek NC (2014b) Arabidopsis STAY-GREEN2 is a negative regulator of chlorophyll degradation during leaf senescence. Mol Plant 7:1288–1302
Seal AG (1983) DNA variation in Festuca. Heredity 50:225–236
Sharma P, Dubey RS (2005) Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J Plant Physiol 162:854–864
Simova-Stoilova L, Vaseva I, Grigorova B, Demirevska K, Feller U (2010) Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol Biochem 48:200–206
Sleper DA (1985) Breeding tall fescue. Plant Breed Rev 3:313–342
Sofo A, Dichio B, Xiloyannis C, Masia A (2005a) Antioxidant defences in olive trees during drought stress: changes in activity of some antioxidant enzymes. Func Plant Biol 32:45–53
Sofo A, Tuzio AC, Dichio B, Xiloyannis C (2005b) Influence of water deficit and rewatering on the components of the ascorbate-glutathione cycle in four interspecific Prunus hybrids. Plant Sci 162:403–412
Stange C, Prehn D, Arce-Johnson P (1998) Isolation of Pinus radiate genomic DNA suitable for RAPD analysis. Plant Mol Biol Rep 16:1–8
Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development. Sinauer Associates, Inc. p 761
Wang JZ, Cui LJ, Wang Y, Li JL (2009) Growth, lipid peroxidation and photosynthesis in two tall fescue cultivars differing in heat tolerance. Biol Plant 53:237–242
Wei Q, Guo Y, Kuai B (2011) Isolation and characterization of a chlorophyll degradation regulatory gene from tall fescue. Plant Cell Rep 30:1201–1207
Yamada T (2011) Festuca. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Springer, p 318
Yang Z, Miao Y, Yu J, Liu J, Huang B (2014) Differential growth and physiological responses to heat stress between two annual and two perennial cool-season turfgrasses. Sci Hort 170:75–81
Acknowledgments
We thank Dr. J. Razmjoo (University of Isfahan, Iran) for providing tall fescue cultivars. We are grateful to Dr. M. Ghasemi and Miss. S. Esmaili and Mrs. F. Sfandiari (Shiraz University, Iran) for their help during the experiments. My special thanks to Marta Bjornson (University of California, Davis, USA) for reading and revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Rights and permissions
About this article
Cite this article
Sarmast, M.K., Salehi, H. & Niazi, A. Biochemical differences underlie varying drought tolerance in four Festuca arundinacea Schreb. genotypes subjected to short water scarcity. Acta Physiol Plant 37, 192 (2015). https://doi.org/10.1007/s11738-015-1942-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1942-4