Abstract
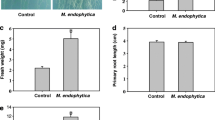
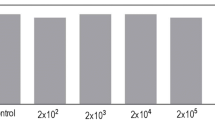
A model system of sand, comprising Arabidopsis plants inoculated with Aeromonas punctata PNS-1 strain, was used to evaluate the bacterial effect in modulation of plant root structure at second-order lateral root level. In MS media, the root morphogenesis was changed only at first-order lateral root level when inoculated with PNS-1 strain. Inoculation with PNS-1 strain was subjected to significant (P < 0.01) increase in primary root length and lateral root density in both MS and sand system. However, this strain modulated the root structure in the sand environment in a complex manner that may be helpful for incitation of the plant–microbe interaction close to natural environment. In order to determine whether this change in root morphology was due to bacterial auxin, Arabidopsis transgenic line (DR5:GUS) was used to reveal the change in homeostasis of endogenous auxin. In PNS-1 inoculated transgenic seedlings of Arabidopsis plant (DR5:GUS), endogenous auxin in primary root apices and lateral roots was enhanced. For confirmation, PNS-1 was evaluated for auxin production in vitro, showed an increase in auxin production after supplementation of l-tryptophan. The presence of ACC deaminase activity in PNS-1 showed its possible involvement in primary root elongation. In the present study Aeromonas punctata PNS-1 is the potential candidate for triggering the change in root morphogenesis of Arabidopsis thaliana with the involvement of auxin and ACC deaminase production.





Similar content being viewed by others
References
Akhtar S, Ali B (2011) Evaluation of rhizobacteria as non-rhizobial inoculants for mung beans. AJCS 5(13):1723–1729
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Quantification of indole-3-acetic acid from plant associated Bacillus spp. and their phytostimulatory effect on Vigna radiata (L.). World J Microbiol Biotechnol 25:519–526
Barbieri P, Galli E (1993) Effect on wheat root development of inoculation with an Azospirillum brasilense mutant with altered indole-3-acetic-acid production. Res Microbiol 144:69–75
Blaha D, Prigent-Combaret C, Mirza MS, Moenne-Loccoz Y (2006) Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS inphytobeneficial and pathogenic proteobacteria and relation with strain biogeography. FEMS Microbiol Ecol 56:455–470
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett M (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8:165–171
Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci Soc Am J 63:1670–1680
Chapman N, Whalley WR, Lindsey K, Miller AJ (2011) Water supply and not nitrate concentration determines primary root growth in Arabidopsis. Plant Cell Environ 34:1630–1638
Cohen JD, Slovin JP, Hendrickson AM (2003) Two genetically discrete pathways convert tryptophan to auxin: more redundancy in auxin biosynthesis. Trends Plant Sci 8:197–199
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-Gonzalez C, Caballero-Mellado J, Aguirre J, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y (2001) Responses of agronomically important crops to inoculation with Azospirillum. Austr J Plant Physiol 28:871–879
Duan J, Muller KM, Charles TC, Vesely S, Glick BR (2006) 1-Aminocyclopropane-1-carboxylate (ACC)deaminase genes in Rhizobia: Isolation, characterization and regulation. Proceedings of the 7th International PGPRWorkshop, Amsterdam
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Gamalero E, Martinotti MG, Trotta A, Lemanceau P, Berta G (2002) Morphogenetic modifications induced by Pseudomonas fluorescens A6RI and Glomus mosseae BEG12 in the root system of tomato differ according to plant growth conditions. New Phytol 155:293–300
Gamalero E, Fracchia L, Cavaletto M, Garbaye J, Frey-Klett P, Varese GC, Martinotti MG (2003) Characterization of functional traits of two fluorescent pseudomonads isolated from basidiomes of ectomycorrhizal fungi. Soil Biol Biochem 35:55–65
Gamalero E, Trotta A, Massa N, Copetta A, Martinotti MG, Berta G (2004) Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 14:185–192
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, Mcconkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26(5):227–242
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Jones DL, Dennis PG, Owen AG, Paw VH (2003) Organic acid behavior in soils-misconceptions and knowledge gaps. Plant Soil 248:31–41
Kapulnik Y, Okon Y, Henis Y (1985) Changes in root morphology of wheat caused by Azospirillum inoculation. Can J Microbiol 31:881–887
Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J (2000) Indole-3-acetic acid: a reciprocal signaling molecule in bacteria–plant interactions. Trends Microbiol 8:298–300
Larcher M, Muller B, Mantelin S, Rapior S, Cleyet-Marel J-C (2003) Early modifications of Brassica napus root system architecture induced by a plant growth-promoting Phyllobacterium strain. New Phytol 160:119–125
Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R (2006) Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol 47:788–792
Lima JE, Kojima S, Takahashi H, von-Wiren N (2010) Ammonium triggers lateral root branching in Arabidopsis in an Ammonium Transporter1;3-dependent manner. Plant Cell 22:3621–3633
Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto- Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129:244–256
Lopez-Bucio J, Campos-Cuevas JC, Hernandez-Calderon E, Velasquez-Becerra C, Farias-Rodriguez R, Macias-Rodriguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact 20:207–217
Lucas M, Guedon Y, Jay-Allemand C, Godin C, Laplaze L (2008) An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS ONE 3(11):1–13
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Eviron 28:67–77
Malamy J, Benfey P (1997) Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci 2:390–396
Mastretta C et al (2006) Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnol Gen Eng Rev 23:175–207
Mayak S et al (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Nagatsu T, Yagi K (1966) A simple assay of monoamine oxidase and d-amino acid oxidase by measuring ammonia. J Biochem 60:219–221
Pare PW, Farag MA, Krishnamachari V, Zhang H, Ryu CM, Kloepper JW (2005) Elicitors and priming agents initiate plant defense responses. Photosynth Res 85:149–159
Patten C, Glick B (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Cnd J Microbiol 47:77–80
Persello-Cartieaux F, Nussaume L, Robaglia C (2003) Tales from the underground: molecular plant-rhizobia interactions. Plant, Cell Environ 26:189–199
Ryu RJ, Patten CL (2008) Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in Enterobacter cloacae UW5. J Bacteriol 190:7200–7208
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. LSMR 21:1–30
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of growth-promoting rhizobacteria containing ACCdeaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42:155–159
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17:2230–2242
Taghavi S et al (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar. Appl Environ Microbiol 75:748–757
Ulmasov T, Murfett J, Hagen G, Guilfoyle T (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wang C, Ramette A, Punjasamarnwong P, Zala M, Natsch A, Moenne-Loccoz Y, Defago G (2001) Cosmopolitan distribution of phlD-containing dicotyledonous crop-associated pseudomonads of worldwide origin. FEMS Microbiol Ecol 37:105–116
Acknowledgments
The Higher Education Commission of Pakistan is acknowledged for providing funds to Atia Iqbal (IRSIP No.1-8/HEC/HRD/2009/514) to visit (Department of Molecular Genetics and Cell Biology, university of Chicago) to carry out this study. We are highly obliged to Jocelyn E. Malamy for her assistance and providing lab facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Li.
Rights and permissions
About this article
Cite this article
Iqbal, A., Hasnain, S. Aeromonas punctata PNS-1: a promising candidate to change the root morphogenesis of Arabidopsis thaliana in MS and sand system. Acta Physiol Plant 35, 657–665 (2013). https://doi.org/10.1007/s11738-012-1106-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1106-8