Abstract
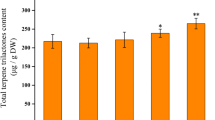
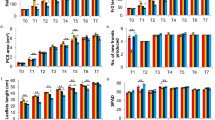
In this study the influence of gibberellic acid (GA3) on plastidic and cytosolic terpenoids and on two key enzymes, 1-deoxy-d-xylulose-5-phosphate synthase (DXS) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), for terpenoid biosynthesis was compared in vegetative cannabis plants. Treatment with GA3 resulted in a decrease of DXS activity in comparison with the control plants. The amount of chlorophylls a, b and total carotenoids declined when plants treated by GA3 in a concentration dependent manner. The α-tocopherol content of cannabis plants decreased in 50 μM GA3 treatment and increased in 100 μM GA3 treatment. Exogenous GA3 caused an increase in HMGR activity. Concomitant with this result, the amount of squalene and phytosterols increased with GA3 treatment. The amount of THC and CBD did not change at 50 μM GA3 treatment, but applying of 100 μM GA3 increased THC and CBD content in leaf plant in comparison with control plants. GA3 treatment declined number and percentage of monoterpenes in treated plants. Also the number of sesquiterpenes decreased in response to GA3 treatment but among the remainder of them, the amount of some sesquiterpenes decreased and some sesquiterpenes increased with GA3 treatment. Our results showed that GA3 treatment had opposite effect on primary terpenoid biosynthesis by the plastidic 2C-methyl-d-erythritol 4-phosphate (MEP) and mevalonate (MVA) pathways. But secondary terpenoids showed different response to GA3 treatment probably due to interference of two biosynthetic pathways in their formation.








Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- GA3 :
-
Gibberellic acid
- THC:
-
Δ9-Tetrahydrocannabinol
- HMGR:
-
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- DXS:
-
1-Deoxy-d-xylulose 5-phosphate synthase
- MEP:
-
2-Methyl-d-erythritol-4-phosphate
- MVA:
-
Mevalonate
References
Abdul Jaleel C, Gopi R, Manivannan P, Sankar B, Kishorekumar A, Panneerselvam R (2007) Antioxidant potentials and ajmalicine accumulation in Catharanthus roseus after treatment with gibberellic acid. Colloids Surf B Biointerf 60:195–200
Bora RK, Sarma CM (2006) Effect of gibberellic acid and cycocel on growth, yield and protein content of pea. Asi J Plant Sci 5:324–330
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodrıguez D (2004) Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. J Plant Growth Regul 23:44–53
Crowell EF, McGrath JM, Douches DS (2008) Accumulation of vitamin E in potato (Solanum tuberosum) tubers. Transgenic Res 17:205–217
Crozier A, Kamiya Y, Bishop G, Yokota T (2000) Biosynthesis of hormones and elicitor molecules. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. Amer Soc Plant Physiol, Rockville, pp 850–929
Est’evez JM, Cantero A, Reindl A, Reichler S, Le’on P (2001) 1-Deoxy-d-Xylulose 5-phosphate synthase, limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909
Fellermeier M, Eisenreich W, Bacher A, Zenk MH (2001) Biosynthesis of Cannabinoids: incorporation experiments with 13C-labeled glucoses. Eur J Biochem 268:1596–1604
Kanno Y, Otomo K, Kenmoku H, Mitsuhashi W, Yamane H, Oikawa H, Toshima H, Matsuoka M, Sassa T, Toyomasu T (2006) Characterization of a rice gene family encoding type-A diterpene cyclases. Biosci Biotechnol Biochem 70:1702–1710
Kato-Emori S, Higashi K, Hosoga K, Kobayashi T, Ezura H (2001) Cloning and characterization of the gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase in melon (Cucumis melo L. reticulatus). Mol Genet Genomics 265:135–142
Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Plant Biol 100:6866–6871
Lange BM, Ghassemian M (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51:925–948
Lichtenthaler HK, Rohmer M, Schwender J (1997) Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol Plant 101:643–652
Misra AN, Biswal UC (1980) Effect of phytohormones on chlorophyll degradation during aging of chloroplasts in vivo and in vitro. Protoplasma 105:1–8
Monge E, Aguirre R, Blanco A (1994) Application of paclobutrazol and GA3 to adult peach trees: effects on nutritional status and photosynthetic pigments. J Plant Growth Regul 13:15–19
Olszewski N, Sun T, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14:S61–S80
Panfili G, Fratianni A, Irano M (2003) Normal phase high-performance liquid method for the determination of tocopherols and tocotrienols in cereals. J Agric Food Chem 51:3940–3944
Pellegrini M, Marchei E, Pacifici R, Pichini S (2005) A rapid and simple procedure for the determination of cannabinoids in hemp food products by gas chromatography–mass spectrometry. J Pharma Biomed Anal 36:939–946
Querol J, Besumbes O, Lois LM, Boronat SO (2001) A fluorometric assay for the determination of 1-deoxy-xylulose 5-phosphate synthase activity. Anal Biochem 296:101–105
Razem FA, Baron K, Hill RD (2006) Turning on gibberellin and abscisic acid signaling. Curr Opin Plant Biol 9:454–459
Rodriguez-Concepcion M (2006) Early steps in isoprenoid biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochem Rev 5:1–15
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Russell DW, Davidson H (1982) Regulation of cytosolic HMG-CoA reductase activity in pea seedlings: contrasting responses to different hormones, and hormone product interaction, suggest hormonal modulation of activity. Biochem Biophys Res Commun 104:1537–1543
Szymanska R, Kruk J (2008) α-Tocopherol content and isomers’ composition in selected plant species. Plant Physiol Biochem 46:29–33
Toroser D, Huber SC (1998) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase kinase and sucrose-phosphate synthase kinase activities in cauliflower florets: Ca2+ dependence and substrate specificities. Arch Biochem Biophys 355:291–300
van den Berg H, Faulks R, Granado HF, Hirschberg J, Olmedilla B, Sandmann G, Southon S, Stahl W (2000) The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric 80:880–912
Yim KO, Kwon YW, Bayer DE (1997) Growth responses and allocation of assimilates of rice seedlings by paclobutrazol and gibberellin treatment. J Plant Growth Regul 16:35–41
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Sowinski.
Rights and permissions
About this article
Cite this article
Mansouri, H., Asrar, Z. & Amarowicz, R. The response of terpenoids to exogenous gibberellic acid in Cannabis sativa L. at vegetative stage. Acta Physiol Plant 33, 1085–1091 (2011). https://doi.org/10.1007/s11738-010-0636-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0636-1
Keywords
Profiles
- Hakimeh Mansouri View author profile