Abstract
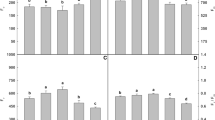
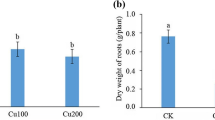
The present study employed a sand culture experiment with three levels of zinc viz., 0.065 (control), 65.0 and 130 mg l−1 Zn (excess) as zinc sulfate, respectively, in sugarcane (Saccharum spp.), cultivar CoLk 8102. The results indicated growth depression, dark green leaves, decreased root number and length and sharp depression in mitotic activity of roots due to high doses of Zn (65 and 130 mg l−1); effects were significant at 130 mg l−1 Zn supply. The endogenous ion contents measurements revealed roots to be the major sink for excess Zn with lower amounts in leaves of sugarcane plants. High level of Zn decreased total phosphorus in leaves and increased it in roots. Fe and Cu content decreased, while, Mn increased in sugarcane plants due to high Zn in the growing medium. Plants experienced oxidative stress when exposed to higher levels of zinc. Biochemical investigations indicated high level of hydrogen peroxide, malondialdehyde contents with high chlorophyll a, b and carotenoids contents and activity of superoxide dismutase, catalase and peroxidase enzymes under high Zn conditions. These findings confirm suggest that excess Zn adversely affects root growth and mitotic efficiency, enhances chromosomal aberrations and increases growth and nutrient accumulation abnormalities, as well as oxidative stress.

Similar content being viewed by others
Abbreviations
- EDTA:
-
Ethylenediaminetetra-acetic acid
- HCl:
-
Hydrochloric acid
- AAs:
-
Atomic absorption spectrophotometer
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- OD:
-
Optical density
- TCA:
-
Trichloroacetic acid
- ROS:
-
Reactive oxygen species
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agarwala SC, Chatterjee C, Sharma CP, Nautiyal N (1985) Copper nutrition of sugar beet. J Exp Bot 36:881–888
Alloway BJ (1995) Heavy metals in soils, 2nd edn. Blackie Academic Professional, London
Amer SM, Ali ME (1968) Cytological effects of pesticides. IV. Mitotic effects of some phenols. Cytologia 34:533–540
Arduini I, Godbold DL, Onnis A (1994) Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pineaster seedlings. Physiol Plant 92:675–680
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1987) The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2 scavenging in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, USA, pp 715–735
Badr A (1983) Mitodepressive and chromotoxic activities of two herbicides in Allium cepa. Cytologia 48:451–457
Barber SA (1995) Soil nutrient bioavailability, 2nd edn. Wiley, New York
Beauchamp C, Fridovich I (1971) Superoxide dismutases: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beu SL, Sachwarz OJ, Hughes KW (1976) Studies of the herbicide paraquat. I. Effects on cell cycle and DNA synthesis in Vicia faba. Can J Genet Cytol 18:93–99
Boawn LC, Rasmussen PE (1971) Crop response to excessive zinc fertilization of alkaline soil. Agron J 63:874–876
Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv. Apollo). J Exp Bot 51:945–953
Bowen JE (1981) Kinetics of active uptake of boron, zinc, copper and manganese in barley and sugarcane. J Plant Nutr 3:215–223
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Carroll MD, Loneragan JF (1968) Response of plant species to concentrations of zinc in solution. I. Growth and zinc content of plants. Aust J Agric Res 19:859–868
Chaney RL (1993) Zinc phytotoxicity. In: Robson AD (ed) Zinc in soil and plants. Kluwer, Dordrecht, pp 135–150
Chaoui A, Mazhoudi S, Ghorbal MH, Elferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chatterjee C, Jain R, Dube BK, Nautiyal N (1998) Use of carbonic anhydrase for determining zinc status of sugarcane. Trop Agric (Trinidad) 75:1–4
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Collins JC (1981) Zinc. In: Lepp NW (ed) Effects of heavy metal pollution on plants. Applied Science Publishers, London, pp 145–169
Davies BE (1993) Radish as an indicator plant for derelict land–uptake of zinc at toxic concentrations. Commun Soil Sci Plant Anal 24:1883–1895
Dudka S, Piotrowska M, Terelak H (1996) Transfer of cadmium, lead, and zinc from industrially contaminated soil to crop plants: a field study. Environ Pollut 94:181–188
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Foy CD, Chaney RL, White MC (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29:511–566
Foyer CH, Harbinson J (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 1–42
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Hewitt EJ (1983) Essential and functional methods in plants. In: Robb DA, Pierpoint WS (eds) Metals and micronutrients: uptake and utilization by plants. Academic Press, New York, pp 313–315
Jain R, Srivastava S, Madan VK (2000) Influence of chromium on growth and cell division of sugarcane. Indian J Plant Physiol 5:228–231
Jain R, Shrivastava AK, Solomon S, Srivastava S (2008) Influence of excess copper on sugarcane metabolism and nutrient composition. Indian J Plant Physiol 13:84–87
Lee CR, Page NR (1967) Soil factors influencing the growth of cotton following peach orchards. Agron J 59:237–240
Lerda D (1992) The effects of lead on Allium cepa L. Mutat Res 281:89–92
Lin CC, Kao CH (2001) Cell wall peroxidase activity, hydrogen peroxide level and Na–Cl inhibited root growth of rice seedlings. Plant Soil 230:135–143
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–375
Lozano-Rodriguez E, Hernandez LE, Bonay P, Carpena-Ruiz RO (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
Luck H (1963) Peroxidase. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Academic Press, New York, pp 895–897
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Monk LS, Fagerstedt KY, Crawford RMM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76:456–459
Nandi S (1985) Studies on the cytogenetic effect of some mercuric fungicides. Cytologia 50:921–926
Ouariti O, Gouia H, Ghorbal MH (1997) Responses of bean and tomato plants to cadmium: growth, mineral nutrition and nitrate reduction. Plant Physiol Biochem 35:347–354
Pahlsson AMB (1989) Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants: literature review. Water Air Soil Pollut 47:287–319
Panse VG, Sukhatme P (1985) Statistical methods for agricultural workers, 4th edn. ICAR, New Delhi
Richardson MD, Hoveland CS, Bacon CW (1993) Photosynthesis and stomatal conductance of symbiotic and nonsymbiotic tall fescue. Crop Sci 33:145–149
Ross SM (1994) Sources and forms of potentially toxic metal in soil–plant systems. In: Ross SM (ed) Toxic metals in soil-plant systems. Wiley, New York, pp 3–25
Saffar A, Bagherieh Najjar MB, Mianabadi M (2009) Activity of antioxidant enzymes in response to cadmium in Arabidopsis thaliana. J Biol Sci 9:44–50
Sagardoy R, Morales F, Lopez-Millan AF, Abadıa A, Abadıa J (2009) Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol 11:339
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco over expressing glyoxalase pathway enzymes grow and viable seeds in zinc-spiked soils. Plant Physiol 140:613–623
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85:85–89
Srivastava S (1995) A modified pretreatment procedure for mitotic studies in sugarcane root tips. Indian J Sugarcane Technol 10:107–110
Staker EV, Cummings RW (1941) The influence of zinc on the productivity of certain New York Peat soils. Soil Sci Soc Am Proc 6:207–214
Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A (2005) Comparative study of responses in four Datura species to a zinc stress. Chemosphere 59:1005–1013
Van Assche F, Clijsters H (1986) Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentration of zinc: effect on ribulose-1, 5-biphosphate carboxylase/oxygenase. J Plant Physiol 125:355–360
Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P (2004) Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett 576:306–312
Wallace A, Romney EM (1977) Roots of higher plants as a barrier to translocation of some metals to shoots of plants. In: Biological implications of metals in the environment. Proceedings of 15th Ann Hanford Life Science Symposium Richland, Washington, 29 September to 10 October, pp 370–379
Weckx JEJ, Clijsters HMM (1997) Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 35:405–410
Zarcinas BA, Pongsakul P, McLaughlin MJ, Cozens G (2004) Heavy metals in soils and crops in Southeast Asia 2. Thailand Environ Geochem Health 26:359–371
Zenk MH (1996) Heavy metal detoxification in higher plants: a review. Gene 179:21–30
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
About this article
Cite this article
Jain, R., Srivastava, S., Solomon, S. et al. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiol Plant 32, 979–986 (2010). https://doi.org/10.1007/s11738-010-0487-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0487-9