Abstract
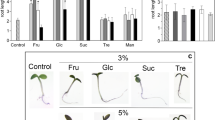
A dynamic cytoskeleton able to recognize and respond to both abiotic and biotic stimuli is necessary for the proper functioning of a living cell. The cytoskeleton is involved in cell growth and division, maintenance of cell shape, cytoplasmic streaming, and organelle movements. Numerous studies have focused on the relationships between sugar metabolism, sugar signaling, and the cytoskeleton in yeast and animal cells. Data on such connections in plants are scarce. In the present study we investigated the effects of exogenously delivered sugars on the plant actin cytoskeleton. Detached Arabidopsis thaliana leaves were incubated with sugars for 2 days and the cytoskeleton was visualized using fluorescent-labeled phalloidin. Glucose and sucrose did not influence the pattern of the actin cytoskeleton. In contrast, mannose caused the disappearance of filamentous structures and generated actin foci. The symptoms started to be visible after 24 h of the exposure to mannose. The effect did not occur in Nicotiana tabacum mesophyll cells. This insensitivity was probably due to the presence of phosphomannose isomerase in tobacco cells. Mannose is commonly used as a selection marker for the transformation of plants lacking the enzymes responsible for the metabolism of mannose-6-phosphate. Exposure to this hexose has been linked with DNA fragmentation and a release of cytochrome c from mitochondria. Both responses are treated as features of programmed cell death. However, in our experiments no DNA laddering was observed in mannose-treated Arabidopsis leaves.




Similar content being viewed by others
Abbreviations
- PCD:
-
Programmed cell death
- PMI:
-
Phosphomannose isomerase
References
Anielska-Mazur A, Bernaś T, Gabryś H (2009) In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses. BMC Plant Biol 9:64. doi:10.1186/1471-2229-9-64
Balasubramanian R, Karve A, Kandasamy M, Meagher RB, Moore B (2007) A role for F-actin in hexokinase-mediated glucose signaling. Plant Physiol 145:1423–1434
Banaś AK, Gabryś H (2007) Influence of sugars on blue light-induced chloroplast movements. Plant Signal Behav 4:221–230
Barb AW, Mason Pharr D, Williamson JD (2003) A Nicotiana tabacum cell culture selected for accelerated growth on mannose has increased expression of phosphomannose isomerase. Plant Sci 165:639–648
Baskin TI, Remillong EL, Wilson JE (2001) The impact of mannose and other carbon sources on the elongation and diameter of the primary root of Arabidopsis thaliana. Funct Plant Biol 28:481–488
Ciereszko I, Kleczkowski LA (2002) Glucose and mannose regulate the expression of a major sucrose synthase gene in Arabidopsis via hexokinase-dependent mechanisms. Plant Physiol Biochem 40:907–911
Ciereszko I, Johansson H, Hurry V, Kleczkowski LA (2001) Phosphate status affects the gene expression protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta 212:598–605
Danon A, Delorme V, Mailhac N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38:647–655
Datta R, Chourey PS (2001) Sugar-regulated control of α-tubulin in maize cell suspension culture. Plant Cell Rep 20:262–266
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Gawal NJ, Jarret RL (1991) A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol Biol Rep 9:262–266
Gibson S (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102
Grabalska M, Malec P (2004) Blue light-induced chloroplast reorientations in Lemna trisulca L. (Duckweed) are controlled by two separable cellular mechanisms as suggested by different sensitivity to wortmannin. Photochem Photobiol 79:343–348
Ha TS (2006) High glucose and advanced glycosylated end-products affect the expression of a α-actinin-4 in glomerular epithelial cells. Nephrology 11:435–441
Herold A, Lewis DH (1977) Mannose and green plants: occurrence, physiology and metabolism, and use as a tool to study the role of orthophosphate. New Phytol 79:1–40
Holtgräwe D, Scholz A, Altman B, Scheibe R (2005) Cytoskeleton-associated, carbohydrate-metabolizing enzymes in maize identified by yeast two-hybrid screening. Physiol Plant 125:141–156
Kandasamy MK, Meagher RB (1999) Actin-organelle interaction: association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskelet 44:110–118
Krzeszowiec W, Gabryś H (2007) Phototropin mediated relocation of myosins in Arabidopsis thaliana. Plant Signal Behav 5:333–336
Malec P, Rinaldi RA, Gabryś H (1996) Light-induced chloroplast movements in Lemna trisulca L. Identification of the motile system. Plant Sci 120:127–137
Maleszewski S, Ciereszko I, Skowrońska A, Mieczejko E, Kozłowska-Szerenos B (2004) Changes induced by low oxygen concentration in photosynthetic and respiratory CO2 exchange in phosphate-deficient bean leaves. Biol Plant 48:401–405
Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K (2001) Effects of sugar on vegetative developmental and floral transition in Arabidopsis. Plant Physiol 127:252–261
Pedley KC, Jones GE, Magnani M, Rist RJ, Naftalin RJ (1993) Direct observation of hexokinase translocation in stimulated macrophages. Biochem J 291:515–522
Pego JV, Weisbeek PJ, Smeekens SCM (1999) Mannose inhibits Arabidopsis germination via a hexokinase-mediated step. Plant Physiol 119:1017–1024
Pollard T (1984) Polymerization of ADP-actin. J Cell Biol 99:769–777
Price J, Li T, Kang SG, Na JK, Jang J (2003) Mechanism of glucose signaling during germination of Arabidopsis. Plant Physiol 132:1424–1438
Reed J, Privalle L, Powell ML, Meghji M, Dawson J, Dunder E, Suttie J, Wenck A, Launis K, Kramer C, Chang Y-F, Hansen G, Wright M (2001) Phosphomannose isomerase: an efficient selectable marker for plant transformation. In Vitro Cell Dev Biol Plant 37:127–132
Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20:4513–4521
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14:S185–S205
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Santagelo GM (2006) Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70:253–282
Staiger CJ, Franklin-Tong VE (2003) The actin cytoskeleton is a target of the self-incompatibility response in Papaver rhoeas. J Exp Bot 54:103–113
Stein JC, Hansen G (1999) Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiol 121:71–79
Tlałka M, Gabryś H (1993) Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta 189:491–498
Veselská R, Zitterbart K, Jelinková S, Neradil J, Svoboda A (2003) Specific cytoskeleton changes during apoptosis accompanying induced differentiation of HL-60 myeloid leukemia cells. Oncol Rep 10:1049–1058
Winter H, Huber JL, Huber SC (1998) Identification of sucrose synthase as an actin-binding protein. FEBS Lett 430:205–208
Xiao W, Sheen J, Jang J-Ch (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44:451–461
Zhou X, Hurst RD, Templeton D, Whiteside CI (1995) High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73:372–383
Zhou X, Li C, Dlugosz J, Kapor-Drezgic J, Munk S, Whiteside C (1997) Mesangial cell actin disassembly in high glucose mediated by protein kinase and the polyol pathway. Kidney Int 51:1797–1808
Acknowledgments
This study was supported by PB 1395/B/P01/2007/33 from Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Rights and permissions
About this article
Cite this article
Banaś, A.K., Krzeszowiec, W., Dobrucki, J. et al. Mannose, but not glucose or sucrose, disturbs actin cytoskeleton in Arabidopsis thaliana leaves. Acta Physiol Plant 32, 773–779 (2010). https://doi.org/10.1007/s11738-010-0462-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0462-5