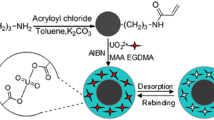
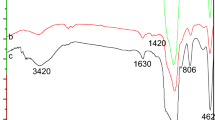
Abstract
In recent years, resins prepared via molecular imprinting technology have received considerable attention owing to their recognition and selective adsorption. This paper deals with the comparative investigation between a uranyl sulphate imprinted ion-exchange based on self-assembling molecular imprinting technology and two kinds of commercial uranium resins (the medium pore resin D263 and strong base resin 201 × 7). The studies were focused on their kinetics performance, adaptability toward pH, and performance of saturation and elution in laboratory-scale column. The results show that the imprinted ion exchange resin has the fast kinetics, high adaptability toward pH, and good adsorption and elution performance.
Similar content being viewed by others
References
Vlatakis G, Andersson L I, Muller R, Mosbach K. Drug assay using antibody mimics made by molecular imprinting. Nature, 1993, 361: 645–647
Wulff G, Sarhan A. Enzyme-analogue built polymers and their use for the resolution of racemates. Tetrahedron Lett, 1973, 44: 4,329–4,332
Joshi V P, Karode S K, Kulkarmi M G, Mashelkar R A. Novel separation strategies based on molecularly imprinted adsorbents. Chem Eng Sci, 1999, 53: 2,271–2,284
Joshi V P, Kulkarni M G, Mashelkar R A. Enhancing adsorptive separations by molecularly imprinted polymers: Role of imprinting techniques and system parameters. Chem Eng Sci, 2000, 55: 1,509–1,522
Martha S V, David A S. Molecular imprinting made easy. J Am Chem Soc, 2004, 126: 7,827–7,833
Matsui J, Nichills I A, Karube I, Mosbach K. Carbon-carbon bond formation using substrate selective catalytic polymers prepared by molecular imprinting: An artificial class II aldolase. J Org Chem, 1996, 61: 5,414–5,417
Dickert F L, Besnbook H, Tortschanoff M. Molecular imprinting through van der waals interactions: Fluorescence detection of PAHs in water. Adv Mater, 1998, 10: 149–155
Kim H, Kaczmarski K, Guiochon G. Intraparticle mass transfer kinetics on molecularly imprinted polymers of structural analogues of a template. Chem Eng Sci, 2006, 61: 1,127–1,137
Fujiwara I, Maeda M, Takagi M. Preparation of ferrocyanide-imprinted pyridine-carrying microspheres by surface imprinting polymerization. Anal Sci, 2003, 19: 617–620
Cui A H, Amarjit S, David L K. Enzyme-based molecular imprinting with metals. Biomacromolecules, 2002, 3: 1,353–1,358
Guo B H, Kim Y K, Jardine P M. Sorption and binary exchange of nitrate, sulfate, and uranium on an anion-exchange resin. Environ Sci Technol, 2004, 38: 3,184–3,188
Song Y, Wang Y, Wang L, Song C, Yang Z, Zhao A. Recovery of uranium from carbonate solutions using strongly basic anion exchanger (4): Column operation and quantitative analysis. React Funct Polym, 1999, 39: 245–252
Unsworth E R, Cook J M, Hill S J. Determination of uranium and thorium in natural waters with a high matrix concentration using solid-phase extraction inductively coupled plasma mass spectrometry. Anal Chim Acta, 2001, 442: 141–146
Aly H M. Chromatographic studies on the uptake of cobalt and uranium from nitrate solution on Al-13-phosphatoantimonic. Sep Purif Technol, 2001, 24: 413–417
Barton C S, Stewart D I, Morris K, Bryant D E. Performance of three resin-based materials for treating uranium-contaminated groundwater within a PRB. J Hazard Mater, 2004, B116: 191–204
Liu Y C, Zhang X W, Liu H J, Xu W J. Study on synthesis of uranyl sulphate imprinted ion exchange resin and its recognition characteristics. Journal of Chemical Engineering of Chinese Universities, 2006, 20: 510–514 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Xu, W., Xu, W. et al. Comparison between two commercial uranium resins and a uranyl sulphate imprinted resin based on self-assembling MIT. Front. Chem. Eng. China 1, 327–331 (2007). https://doi.org/10.1007/s11705-007-0059-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11705-007-0059-8