Abstract
Despite standardized surgical technique and peri-operative care, metabolic outcomes of bariatric surgery are not uniform. Adaptive changes in brain function may play a crucial role in achieving optimal postbariatric weight loss. This review follows the anatomic-physiologic structure of the postbariatric nutrient-gut-brain communication chain through its key stations and provides a concise summary of recent findings in bariatric physiology, with a special focus on the composition of the intestinal milieu, intestinal nutrient sensing, vagal nerve-mediated gastrointestinal satiation signals, circulating hormones and nutrients, as well as descending neural signals from the forebrain. The results of interventional studies using brain or vagal nerve stimulation to induce weight loss are also summarized. Ultimately, suggestions are made for future diagnostic and therapeutic research for the treatment of obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
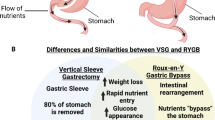
While alterations in intestinal physiology, gut-brain communication, and cerebral connectivity following bariatric surgery (BS) are increasingly recognized as important factors mediating long-term metabolic outcomes [1, 2], the clinical relevance and applicability of recent findings require further investigation. Despite standardized surgical technique and peri-operative care, patients do not evolve uniformly after BS. Excess weight loss (EWL) outcomes after Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) vary widely (37.6–94.4%), and existing models of EWL prediction based on demographic data have been shown to be inaccurate [3].
The extent of postbariatric EWL may highly depend on factors that are not regularly assessed during peri-operative clinical care, such as changes in brain connectivity, as documented in studies using functional magnetic resonance imaging (fMRI) [4, 5]. The gastrointestinal (GI) tract senses the chemical, nutritive and volumetric properties of ingested food via a complex gut-brain communication system [6]. As a crucial link between the gut and the brain, the vagus nerve remains an important subject of research in gastrointestinal physiology [1].
This review provides a concise summary of recent findings in bariatric physiology, with a focus on how gut-brain communication pathways are altered following BS and in the context of selected non-surgical experimental weight-loss therapies. The review of specific changes in nutrient sensing in the context of various obesity therapy modalities has the potential to elucidate causes of variability in postbariatric outcomes. The discussion follows an anatomic-physiologic structure along the key stations of nutrient-gut-brain communication to identify potential diagnostic and therapeutic targets for optimizing obesity therapy in the setting of BS. We also explore how integrating biotechnology into BS could amplify the functional brain changes that appear to be crucial for successful weight loss.
Methods
We performed a narrative review to provide in-depth coverage of nutrient sensing and gut-brain communication in surgical and experimental obesity therapy and to synthesize rationales for future research. Each subchapter has been drafted to provide novel insights into physiologic research from different angles. The selection of included articles from the literature aimed to include the most recent and the most relevant articles from preclinical and clinical research, without the application of a systematic bibliographic search strategy. The articles were critically evaluated based on key results, limitations, suitability of the methods used to test the initial hypothesis, and their impact in the field [7]. Subjectivity in study selection is an inherent limitation of narrative reviews, which may influence the interpretation, the translation and the application of published research [8]. Physiologic changes in nutrient sensing and metabolism following BS are emphasized with italics and summarized in Table 1.
Intestinal Nutrient Sensing
Anatomical changes after BS (i.e., RYGB or SG) result in faster delivery of partially undigested nutrients to the jejunum. This, in turn, alters the nutrient-sensing mechanisms controlling gut hormone release (e.g., increased postprandial GLP-1 and PYY release from enteroendocrine cells (EEC)) [9, 10]. A longitudinal study showed that after RYGB, patients decreased their meal size in part by lowering the average size, but not the total number of within-meal ingestive bursts [11]. This suggests that neural circuits responsible for within-meal intestinal nutrient sensing are crucial for postbariatric changes in ingestive behavior and demonstrates the rapidity of the gut-brain feedback loop.
A multitude of GI cell types participate in nutrient chemo-sensing and produce signaling molecules to relay this information to other organs. Their composition and distribution across the GI tract vary considerably, indicating that specific nutrient sensing occurs in a tissue-dependent fashion. EEC and tuft cells constitute the first line of hormonal, neuronal, and immunogenic response to food intake [12,13,14]. Nutrient chemo-sensing occurs in part through multiple G protein-coupled nutrient receptors (GPCRs) expressed on the apical membrane of EEC [15], whereas tuft cells participate in chemosensing thanks to their expression of specific taste receptors [16]. Moreover, tuft cells have been shown to regulate immune responses [17] and convey information to the enteric nervous system, eliciting direct neuromodulatory activities [18, 19].
Metabolic disorders such as obesity correlate with major changes in the number of these cells and their hormonal release [9, 20]. Following BS, profound alterations in the distribution and activity of EEC cells have been described [9, 20]. For example, in a comparison of lean control individuals to patients with obesity pre- and post-BS, an overall reduction in the number of duodenal EEC was observed in patients with obesity, which was partially restored following SG [9]. Interestingly, individuals with obesity had more mucin-producing Goblet cells, underscoring the biased allocation of the intestinal secretory lineage [21]. Gastrin receptors and the bile acid receptors FXR and TGR5 have been described to participate in this process by modulating EEC differentiation [22,23,24] as well as intestinal progenitor proliferation [18, 25,26,27]. Interestingly, these observations suggest that an evaluation of the distribution and activity of secretory cells may provide a valuable readout for proper nutrient chemo-sensing to test the effectiveness of surgical and pharmacological treatment of metabolic disorders.
Summary: Multiple cell populations actively participate in postprandial nutrient chemo-sensing. Their localization and function vary across the GI tract, reflecting an anatomical regionalization in nutrient sensing. Metabolic disorders can alter the representation and function of these cells, leading to improper chemo-sensing. BS appears to be effective in counter-balancing these obesity-related alterations.
Intestinal Milieu
The internal environment of the GI tract is shaped by numerous factors, including diet, digestive secretions, resident microbiota, and fungi [28,29,30,31,32,33]. Recent developments in ingestible, pH-sensitive sampling devices have revealed profound differences in these factors throughout the GI tract in healthy humans [34, 35], which are all liable to change following the anatomic alterations of BS. This may, in turn, contribute to postbariatric metabolic outcomes, such as EWL, or the weight loss-independent amelioration of liver steatosis and type 2 diabetes mellitus (T2DM).
Rodent models of BS have been instrumental in identifying post-surgical changes in the intestinal milieu, particularly in gut microbiota and bile acids, which are interrelated and play emerging roles in metabolic health [36]. For example, a study on Zucker fatty rats found that RYGB causes major shifts in the microbiota across the small and large intestine [37]. Interestingly, transplanting ileal microbiota from RYGB-operated rats to germ-free mice worsened oral glucose tolerance [37], likely due to the generation of metabolites that disrupt the intestinal epithelial barrier and trigger systemic endotoxemia [38]. On the other hand, transplanting colonic microbiota of RYGB-operated rats to germ-free mice improved oral glucose tolerance, possibly by stabilizing the intestinal epithelial barrier through increased bile acid receptor FXR signaling via the generation of secondary bile acids [39]. These findings suggest that the changes in gut microbiota and their associated metabolites after RYGB may have opposing effects on glycemic control, depending on the gut region [40]. For SG, cecal and fecal levels of the primary bile acid cholic acid-7 sulfate (CA7S) have been found to increase in mice and patients after surgery [41]. Moreover, oral administration of CA7S improved glucose tolerance in mice in a TGR5-dependent manner [41]. These findings illustrate how understanding changes in the gut milieu after BS can guide the development of new pharmacological treatments for T2DM. There is also evidence that the gut microbiota plays a role in the reduced food intake [41] and protection from weight gain after RYGB in diet-induced obese rats [42]. In line with this, antibiotic-based depletion of the gut microbiota abrogates the effects of RYGB on energy balance, and fecal transplantation is effective in transferring the metabolic phenotype of RYGB-operated rats to diet-induced obese rats, again mediated by increased FXR signaling [42]. These findings appear to have limited translational value, since transferring human feces after RYGB fails to impact body weight in rodents and in humans [43,44,45]. Interestingly, this intervention does improve oral glucose tolerance in recipients through reduced glucose absorption and increased adipose tissue glucose utilization [43, 44], suggesting that post-RYGB changes in gut microbiota mainly improve glycemic control. Clearly, the impact of RYGB on gut microbiota-host interactions is complex, and more research is needed before an efficient RYGB microbiota-based treatment can be developed for patients with metabolic syndrome.
Beyond bile acids and microbiota, BS affects other factors in the intestinal milieu. In a study of women with T2DM and obesity, samples were collected by endoscopy from the stomach and throughout the small intestine two weeks before and three months after RYGB, providing insight into how glucose metabolites change in the intestinal milieu [46]. As revealed by mass spectrometry analysis, RYGB led to reduced levels of aromatic and branched-chain amino acids, while it increased the metabolism of phenylacetate and degradation of trehalose in the duodenum and jejunum, and degradation of lactose in the ileum [46].
Summary: Preclinical and clinical research is beginning to shed light on the complex changes in the intestinal milieu after BS [47]. Further work is needed to determine how this influences circulating signaling molecules that communicate with peripheral tissues and the brain to improve metabolic health.
Nutrient Sensing Via the Vagus Nerve
The vagus nerve provides parasympathetic innervation of the gastrointestinal tract from the esophagus to the splenic flexure of the colon. It carries efferent signals from the dorsal vagal nucleus in the hindbrain, which are integrated by the enteric nervous system to control smooth muscle contraction and glandular secretion. However, afferent vagal fibers vastly outnumber efferent fibers [48]. Vagal afferent neurons, whose cell bodies reside in the inferior (nodose) ganglion of the vagus nerve in the jugular foramen, carry sensory information from the gut to the nucleus of the solitary tract (NTS) of the hindbrain.
In the stomach, most vagal afferents terminate in intramuscular arrays within the circular and longitudinal muscle layers, as well as in the ganglia of the myenteric plexus. They are thus well-positioned to react to mechanical stretch and tension [49]. A smaller number of vagal afferent fibers project to the gastric mucosa and respond to stroking [50]. In the intestine, most vagal fibers are chemosensitive and end in the mucosa, where they respond to nutrients and gastrointestinal hormones [51, 52]. Recently, new genetic tools – including monosynaptic neural tracing, optogenetics, and chemogenetics – allowed the characterization of vagal afferents in mice that rapidly communicate the presence of nutrients from the gut to the brain. In one study, a novel neuroendocrine cell that communicates the presence of sugars to vagal afferents via glutamate was identified. Because of the synaptic-like nature of this signaling, the authors termed this neuroepithelial unit a “neuropod” [53].
Signaling through the vagal nerve may mediate some of the benefits of BS [54, 55]. In RYGB, only the dorsal and ventral gastric vagal branches supplying the stomach are severed while forming the gastric pouch. However, the vagal branches traveling with the gastroduodenal and superior mesenteric arteries remain intact. Thus, vagal innervation of the intestine, liver, and pancreas can be mostly spared, except for some branches traveling along the lesser curvature of the stomach to reach the proximal duodenum and parts of the pancreas [56]. Evidence from the rat model indicates that preservation of the celiac branches of the vagal nerve enhances weight loss after RYGB [57,58,59].
While gastrointestinal hormones can reach the brain via the circulation to directly regulate feeding behavior, afferent vagal nerve endings contain receptors for many intestinal hormones, and signaling via the vagus nerve may partly account for their effects [60]. Cholecystokinin (CCK), which is released by enteroendocrine cells after ingestion of protein or fat, binds to CCK-A receptors on vagal afferents to suppress further food intake [61]. In rodent models, however, a complete transection of the abdominal vagus nerve has a negligible effect on body weight, although it leads to an increase in meal size, which is compensated by reduced meal frequency [62]. Thus, humoral regulation may be sufficient for long-term control of body weight, with the vagus nerve being more important in the short-term regulation of feeding. However, if a complete transection of all vagal afferents and efferents is performed, it is impossible to identify the contributions of individual neuronal subpopulations, which may have different and opposing effects [63]. In obesity, the sensitivity of vagal afferents to CCK signaling, as well as to mechanical distension of the gut, is reduced [64, 65]. Therefore, targeting specific vagal populations to heighten the response to satiety signals remains a promising therapeutic approach.
Summary: The vagus nerve is instrumental in rapidly responding to satiety signals such as mechanical stretch, intraluminal nutrients and caloric concentration, as well as to paracrine gastrointestinal hormones. However, its role in long-term weight control remains less defined, necessitating a more detailed exploration of vagal signaling and plasticity in obesity and in post-BS conditions.
The role of the Hindbrain in the Hypophagic Effects of Bariatric Surgery
Historically, the mediobasal hypothalamus has been considered the main sensor of the metabolic state and the integrator of effector actions aiming at an optimal body weight [66]. However, the hindbrain and the limbic system are other key brain areas implicated in body weight-regulating mechanisms that serve as alternative targets for weight loss therapies [66]. The NTS and the area postrema (AP) are two adjacent and highly interconnected hindbrain structures that process information on peripheral energy status from circulating signals and branches of the vagus nerve [67]. The presence of fenestrated capillaries and the lack of tight junctions between endothelial cells allow neurons in the AP/NTS to be reached by hormonal and nutrient signals that cannot cross the blood–brain barrier in other brain regions [68, 69]. AP/NTS neurons express a variety of receptors and directly respond to nutrients (e.g., glucose) and several peptides produced peripherally that play a direct role in the regulation of feeding and homeostasis, e.g., GLP-1, amylin, leptin, and ghrelin [70,71,72,73].
The AP/NTS is also a key hub for the integration of pathological modulators of energy balance. Increased neuronal activity in this area is associated not only with physiological satiation, but also with emesis and nausea [74, 75]. AP/NTS neurons project to a number of regions involved in the regulation of feeding [76,77,78,79,80]. Among these connections, many studies highlight the importance of NTS lateral parabrachial nucleus (lPBN) projections [67, 81,82,83,84], where calcitonin gene-related peptide (CGRP) mediation of anorexia and malaise occurs.
Postprandial nausea and vomiting represent potential complications of BS [85]. Although recent clinical neuroimaging and animal studies have explored the effects of BS on forebrain function [86], relatively few studies have focused on the role of the caudal hindbrain and its contribution to BS outcomes. It has been shown in preclinical models that vagal afferent signaling is required for optimal weight loss and diminished fat preference following RYGB [58]. Relatedly, it was demonstrated that in RYGB mice, eating a voluntary meal induced exaggerated expression of the marker of neuronal activation c-Fos in the AP/NTS [87]. Interestingly, a significant portion of the activated neurons in the lPBN expressed CGRP, suggesting the involvement of the hindbrain in the mediation of the food taste-visceral malaise association occurring after surgery [87]. SG also increases nutrient-induced c-Fos expression in the AP/NTS compared to control animals [88]. These results may provide a mechanistic explanation for such post-surgical symptoms as nausea, vomiting, and visceral malaise, which most frequently occur after the consumption of large meals rich in fat or sugar [89].
The gut-generated signals responsible for the modified hindbrain response after RYGB are largely unknown. However, recent research has focussed on the stress-response cytokine growth differentiation factor 15 (GDF15) and its body weight-suppressive effects. GDF15 is an inflammatory biomarker released by various tissues [90,91,92,93,94], and elevated circulating levels of GDF15 are associated with anorexia, malaise, and cachexia in a variety of diseases and physiological states [95,96,97,98,99,100,101,102,103,104,105]. GDF15 acts as a ligand on a highly localized hindbrain G-family α-like receptor [103, 106,107,108,109]. Exogenous administration of GDF15 induces nausea and emesis, suggesting malaise and conditioned food aversion as key components of GDF15-induced anorexia [110,111,112,113].
Interestingly, while circulating GDF15 levels increase only slightly in obesity, they are substantially elevated in patients following RYGB (and, to a lesser extent, SG) [90,91,92,93,94]. Importantly, there was a clear correlation between GDF15 levels and the magnitude of post-BS EWL [90, 93]. In mice treated with GDF15, there was a reduced preference for a high-fat diet [114]. Notably, RYGB-operated rats have increased circulating and portal vein GDF15 levels, and this is negatively correlated with their food intake and body weight [40]. While a separate study also showed increased circulating GDF15 levels and GDF15 protein in the gastric pouch, jejunum, and ileum of RYGB-operated rats [115], more data is needed to establish a causal link between gut-derived GDF15 and the beneficial effects of BS [116, 117].
Summary: Despite being a relatively new and rapidly evolving field, emerging evidence strongly suggests that GDF15 plays a crucial role in inducing hypophagia in various medical conditions by primarily triggering feelings of malaise through signaling to the AP/NTS. However, further pre-clinical and clinical research is needed to fully understand its role in the positive outcomes (e.g., weight loss) and negative effects (i.e., GI malaise) of bariatric surgery.
Weight Loss by Neurostimulation
Based on the strong relationship between BS and brain activity, several studies tried to induce weight loss by direct manipulation of the brain [118]. Cortical functions implicated in the development of obesity include reward, attention, emotional regulation, impulsivity, and motivation. People with obesity show decreased inhibitory control (also known as response inhibition to environmental stimuli) and impaired memory systems [119]. Postbariatric weight loss correlates with functional changes in brain regions associated with cognitive functions altered by obesity [120]. Food cue-based neuroimaging studies after BS have shown increased activation in the dorsolateral prefrontal cortex (DLPFC), which is responsible for inhibitory control, while brain regions responsible for memory and reward processes, such as the hippocampus and insula, are less active [121,122,123]. In an attempt to stimulate weight loss, neuromodulation techniques, such as deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS) of cortical and subcortical locations, neurofeedback and peripheral nerve stimulation/blockade have been applied.
Invasive DBS treatment for weight loss has been limited to only a few patients. These studies aimed to stimulate the hypothalamus or the nucleus accumbens and achieved only clinically irrelevant EWL in humans [124,125,126]. It should be noted that the microlesions in the brain during DBS electrode placement can also evoke metabolic changes [127], and thus, it is not clear whether the altered functionality is the consequence of the stimulation. In addition, the lack of a standardized protocol for the DBS studies makes it hard to evaluate their efficacy [128]. Very recently, a DBS clinical pilot was conducted on two patients, where the electrophysiological signatures of food craving were used to activate implanted DBS to enhance inhibitory control [129]. After six months, the BMI of both subjects decreased by 2–3 kg/m2, which is clearly inferior to what can be achieved with BS [130].
High-frequency repeated TMS is a method used for selective and non-invasive excitatory stimulation of the cortex. A few studies have shown that TMS stimulation of the DLPFC is able to induce weight loss [131,132,133]. Although the results were statistically significant, the achieved weight loss (BMI change < 1 kg/m2 / weight-loss 2–3 kg) is massively smaller than the one observed post-BS. Non-invasive tDCS paradigms aim to interfere with cortical systems at the DLPFC to increase the inhibitory control of ingestion. Based on a meta-analysis, a consistent decrease in food craving and energy intake could be achieved [134, 135]; however, no EWL was seen [136, 137]. Besides external stimulation paradigms, patients can learn to effectively manipulate the activity of circumscribed brain areas with online neurofeedback. These treatments are based on the voluntary regulation of brain activity feedback via real-time EEG [138]. Studies using EEG [139] and fMRI paradigms [140] introduced the possibility of volitional regulation of frontal brain activity. However, research showing a relationship between successful neurofeedback and weight loss is still lacking.
Directly targeting the vagal nerve appears to be more effective for weight loss than cortical manipulation of the gut-brain axis. Vagal nerve stimulation (VNS) involves the surgical implantation of electrodes in the neck and a generator under the skin below the clavicle to provide electrical stimulation to the vagus nerve. The treatment has achieved reduced food intake and EWL proportional to the initial BMI [141, 142]. The opposite approach – electrical vagal nerve blockade – is also established as a potential treatment for obesity. The ReCharge trial investigated a vagal blocking device, which employs electrodes placed on the anterior and posterior vagal trunks close to the oesophageal junction, through which an alternating current was applied to block vagal nerve signaling for 12 h per day. One-year EWL was significantly greater with vagal blocking compared to sham treatment (24.4% vs. 15.9%) [143], and this effect remained quite stable at 2-year follow-up [144], with associated metabolic improvements.
Stimulation of intestinal sensory cells and vagal afferents by electrodes, hybrid or “bionic” tissues may offer a new therapeutic avenue for regulating satiety [145] and treating obesity. The mechanism of action of intestinal stimulation is not completely understood, but is likely manifold, including a) an accelerated intestinal transit which reduces fat absorption and enhances the nutrient-induced release of GLP-1 and GIP in the distal ileum, and b) an increased expression of hypothalamic oxytocin-immunoreactive positive neurons, which may have a direct effect on adipocytes or an indirect effect in promoting lipolysis [146, 147]. Preclinical data are promising: intestinal electrical stimulation has been shown to reduce food intake in rats and pigs and to reduce intestinal absorption and body weight, as well as to improve glucose tolerance and insulin sensitivity in rats [146, 148]. The first human feasibility study included 9 participants, used laparoscopically implanted duodenal stimulation electrodes, and showed effectiveness in optimizing glycemic control and high-density lipoprotein levels [148].
Summary: Neurostimulation of cortical and subcortical areas may lead to weight loss, but the effect size remains relevantly below the observed weight loss achieved after BS. However, stimulation of the peripheral nervous system and hormone-producing cells of the small bowel or intermittent blockade of the abdominal branches of the vagal nerve may open potential pathways to be explored in the quest for non-surgical or adjuvant metabolic therapies.
Future Outlook
The gut-brain hormonal and synaptic communications, together with GI microbiota, are among the key components for the treatment of obesity. The beneficial postbariatric shifts in the intestinal microbiota and their metabolites, of cellular changes in the GI mucosa, of hormonal, cytokine and vagal signal transmission have been demonstrated.
Future studies should focus on investigating how these different pathways interact and on the neurohormonal changes driven by the accelerated delivery of less digested food into the small bowel following BS. Longitudinal studies can track changes in brain activity and connectivity over time, helping to identify causal relationships between brain activity and postbariatric weight loss. Directed network models can identify key hubs in these systems that might be targeted for therapeutic interventions. Additionally, recent advances in tissue clearing and light-sheet microscopy may enable the study of the GI nervous system (e.g., the vagus nerve and enteric nervous system) in toto, allowing for further elucidation of the role of vagal afferents in regulating food intake.
Together with drug-based treatments of obesity, the development of biomedical electronic implants, reaching the micro- and nanoscale, may also provide a therapeutic avenue for the treatment of metabolic disorders [145]. The development of obesity treatments that mimic BS, but are less invasive and are more easily scalable to the eligible patient population, is a high priority. Currently, bionic intestinal stimulators are not available for human use and their potential clinical efficacy is merely hypothetical, and the transferability of preclinical observations to human practice remains to be proven. Limitations and challenges of non-surgical metabolic therapies stem from the chronic nature of obesity and related diseases. Non-surgical weight-loss treatments would ideally need to replicate the pleiotropic physiologic changes and the meal-triggered neurohormonal responses observed after BS. These goals are mainly constrained by the cost of lifelong conservative therapy, by patient compliance, and by the long-term efficacy and side-effect profile of any new treatment. Another limitation is related to the genetic and social factors that may increase the predisposition to obesity, which have not been addressed in this review.
The modified GI anatomy after BS provides a perfect model to study changes at each station of the ingestive signal transmission chain, from the intestinal milieu to the brain. There is a need for further novel and real-time diagnostic studies to better unravel the physiologic mechanisms of BS, with the ultimate goal of broadening preventive and therapeutic strategies for obesity.
References
Berthoud HR, Albaugh VL, Neuhuber WL. Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest. 2021;131(10):e143770. https://doi.org/10.1172/JCI143770
Martinou E, Stefanova I, Iosif E, Angelidi AM. Neurohormonal changes in the gut-brain axis and underlying neuroendocrine mechanisms following bariatric surgery. Int J Mol Sci. 2022;23(6):3339. https://doi.org/10.3390/ijms23063339.
Karpinska IA, Kulawik J, Pisarska-Adamczyk M, et al. Is It Possible to Predict Weight Loss After Bariatric Surgery?-External Validation of Predictive Models. Obes Surg. 2021;31:2994–3004.
Schmidt L, Medawar E, Aron-Wisnewsky J, et al. Resting-state connectivity within the brain’s reward system predicts weight loss and correlates with leptin. Brain Commun. 2021;3:fcab005.
Hu Y, Ji G, Li G, et al. Brain Connectivity, and Hormonal and Behavioral Correlates of Sustained Weight Loss in Obese Patients after Laparoscopic Sleeve Gastrectomy. Cereb Cortex. 2021;31:1284–95.
Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211.
Derish PA, Annesley TM. How to write a rave review. Clin Chem. 2011;57:388–91.
Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104:1086–92.
Wolnerhanssen BK, Moran AW, Burdyga G, et al. Deregulation of transcription factors controlling intestinal epithelial cell differentiation; a predisposing factor for reduced enteroendocrine cell number in morbidly obese individuals. Sci Rep. 2017;7:8174.
Gero D, Steinert RE, Hosa H. Appetite, Glycemia, and Entero-Insular Hormone Responses Differ Between Oral, Gastric-Remnant, and Duodenal Administration of a Mixed-Meal Test After Roux-en-Y Gastric Bypass. Diabetes Care. 2018;41:1295–8.
Gero D, File B, Alceste D, et al. Microstructural changes in human ingestive behavior after Roux-en-Y gastric bypass during liquid meals. JCI Insight. 2021;6.
Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells–how it interacts with enteroendocrine cells. Adv Nutr. 2012;3:8–20.
Kaji I, Kaunitz JD. Luminal chemosensing in the gastroduodenal mucosa. Curr Opin Gastroenterol. 2017;33:439–45.
Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226–37.
Sheng W, Malagola E, Nienhuser H, et al. Hypergastrinemia Expands Gastric ECL Cells Through CCK2R(+) Progenitor Cells via ERK Activation. Cell Mol Gastroenterol Hepatol. 2020;10(434–49):e1.
Bezencon C, Furholz A, Raymond F, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–25.
Schneider C. Tuft cell integration of luminal states and interaction modules in tissues. Pflugers Arch. 2021;473:1713–22.
Middelhoff M, Nienhuser H, Valenti G, et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat Commun. 2020;11:111.
Ten Hove AS, Seppen J, de Jonge WJ. Neuronal innervation of the intestinal crypt. Am J Physiol Gastrointest Liver Physiol. 2021;320:G193–205.
Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110:571–84.
Pelaseyed T, Bergstrom JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–8.
Lund ML, Sorrentino G, Egerod KL, et al. L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes. 2020;69:614–23.
Kim K-S, Peck BCE, Hung Y-H, et al. Vertical Sleeve Gastrectomy Induces Enteroendocrine Cell Differentiation of Intestinal Stem Cells Through Farnesoid X Receptor Activation. bioRxiv. 2021:2021.04.22.440705.
Sorrentino G, Perino A, Yildiz E, et al. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159(956–68):e8.
Chang W, Wang H, Kim W, et al. Hormonal Suppression of Stem Cells Inhibits Symmetric Cell Division and Gastric Tumorigenesis. Cell Stem Cell. 2020;26(739–54):e8.
Cheng CW, Biton M, Haber AL, et al. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell. 2019;178(1115–31):e15.
Ilhan ZE, DiBaise JK, Dautel SE, et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes. 2020;6:12.
Fouladi F, Carroll IM, Sharpton TJ, et al. A microbial signature following bariatric surgery is robustly consistent across multiple cohorts. Gut Microbes. 2021;13:1930872.
Li JV, Ashrafian H, Sarafian M, et al. Roux-en-Y gastric bypass-induced bacterial perturbation contributes to altered host-bacterial co-metabolic phenotype. Microbiome. 2021;9:139.
Westerink F, Huibregtse I, De Hoog M, et al. Faecal Inflammatory Biomarkers and Gastrointestinal Symptoms after Bariatric Surgery: A Longitudinal Study. Inflamm Intest Dis. 2021;6:109–16.
Farup PG, Valeur J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients. 2020;12.
Steinert RE, Rehman A, Souto Lima EJ, et al. Roux-en-Y gastric bypass surgery changes fungal and bacterial microbiota in morbidly obese patients-A pilot study. PLoS One. 2020;15:e0236936.
Shalon D, Culver RN, Grembi JA, et al. Profiling the human intestinal environment under physiological conditions. Nature. 2023;617:581–91.
Folz J, Culver RN, Morales JM, et al. Human metabolome variation along the upper intestinal tract. Nat Metab. 2023;5:777–88.
Petersen N, Greiner TU, Torz L, Bookout A, Gerstenberg MK, Castorena CM, et al. Targeting the gut in obesity: signals from the inner surface. Metabolites. 2022;12(1):39. https://doi.org/10.3390/metabo12010039.
Arora T, Seyfried F, Docherty NG, et al. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017;11:2035–46.
Hankir MK, Seyfried F, Schellinger IN, Schlegel N, Arora T. Leaky gut as a potential culprit for the paradoxical dysglycemic response to gastric bypass-associated ileal microbiota. Metabolites. 2021;11(3):153. https://doi.org/10.3390/metabo11030153.
Hankir MK, Langseder T, Bankoglu EE, et al. Simulating the Post-gastric Bypass Intestinal Microenvironment Uncovers a Barrier-Stabilizing Role for FXR. iScience. 2020;23:101777.
Hankir MK. Building and breaking the gut barrier with bariatric surgery. Cell Stress. 2022;6:17–20.
Ismaeil A, Gero D, Boyle CN, et al. Early Postoperative Exposure to High-Fat Diet Does Not Increase Long-Term Weight Loss or Fat Avoidance After Roux-en-Y Gastric Bypass in Rats. Front Nutr. 2022;9:834854.
Munzker J, Haase N, Till A, et al. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity. Microbiome. 2022;10:96.
Anhê FF, Zlitni S, Zhang SY, et al. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut. 2023;72:460–71.
Yadav J, Liang T, Qin T, et al. Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure. Cell Rep Med. 2023;4:101051.
de Groot P, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69:502–12.
Mendonca Machado N, Torrinhas RS, Sala P, et al. Type 2 Diabetes Metabolic Improvement After Roux-en-Y Gastric Bypass May Include a Compensatory Mechanism That Balances Fatty Acid beta and omega Oxidation. JPEN J Parenter Enteral Nutr. 2020;44:1417–27.
Seyfried F, Phetcharaburanin J, Glymenaki M, et al. Roux-en-Y gastric bypass surgery in Zucker rats induces bacterial and systemic metabolic changes independent of caloric restriction-induced weight loss. Gut Microbes. 2021;13:1–20.
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17.
Powley TL, Hudson CN, McAdams JL, et al. Vagal Intramuscular Arrays: The Specialized Mechanoreceptor Arbors That Innervate the Smooth Muscle Layers of the Stomach Examined in the Rat. J Comp Neurol. 2016;524:713–37.
Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–103.
Page AJ. Vagal afferent dysfunction in obesity: cause or effect. J Physiol. 2016;594:5–6.
Williams EK, Chang RB, Strochlic DE, et al. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–21.
Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5236. https://doi.org/10.1126/science.aat5236.
Gautron L, Zechner JF, Aguirre V. Vagal innervation patterns following Roux-en-Y gastric bypass in the mouse. Int J Obes (Lond). 2013;37:1603–7.
Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591:2357–72.
Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25.
Bueter M, Lowenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–22.
Hao Z, Townsend RL, Mumphrey MB, et al. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg. 2014;24:2145–51.
Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255:294–301.
Brierley DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3:258–73.
Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13:954–8.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594:5791–815.
McDougle M, Quinn D, Diepenbroek C, et al. Intact vagal gut-brain signalling prevents hyperphagia and excessive weight gain in response to high-fat high-sugar diet. Acta Physiol (Oxf). 2021;231:e13530.
Daly DM, Park SJ, Valinsky WC, et al. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–70.
Kentish SJ, O’Donnell TA, Frisby CL, et al. Altered gastric vagal mechanosensitivity in diet-induced obesity persists on return to normal chow and is accompanied by increased food intake. Int J Obes (Lond). 2014;38:636–42.
Berthoud HR, Seeley RJ, Roberts SB. Physiology of Energy Intake in the Weight-Reduced State. Obesity (Silver Spring). 2021;29(Suppl 1):S25–30.
Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309.
Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14:182–94.
Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15:301–20.
Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–2.
Kawatani M, Yamada Y, Kawatani M. Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides. 2018;107:68–74.
Barth SW, Riediger T, Lutz TA, et al. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004;997:97–102.
Zuger D, Forster K, Lutz TA, et al. Amylin and GLP-1 target different populations of area postrema neurons that are both modulated by nutrient stimuli. Physiol Behav. 2013;112–113:61–9.
Bayo J, Fonseca PJ, Hernando S, et al. Chemotherapy-induced nausea and vomiting: pathophysiology and therapeutic principles. Clin Transl Oncol. 2012;14:413–22.
Baker PD, Morzorati SL, Ellett ML. The pathophysiology of chemotherapy-induced nausea and vomiting. Gastroenterol Nurs. 2005;28:469–80.
Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2007;132:44–51.
Horn CC, De Jonghe BC, Matyas K, et al. Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1375–82.
De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol. 2009;296:R902–11.
De Jonghe BC, Holland RA, Olivos DR, et al. Hindbrain GLP-1 receptor mediation of cisplatin-induced anorexia and nausea. Physiol Behav. 2016;153:109–14.
Alhadeff AL, Holland RA, Zheng H, et al. Excitatory Hindbrain-Forebrain Communication Is Required for Cisplatin-Induced Anorexia and Weight Loss. J Neurosci. 2017;37:362–70.
Carter ME, Soden ME, Zweifel LS, et al. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–4.
Chen JY, Campos CA, Jarvie BC, et al. Parabrachial CGRP Neurons Establish and Sustain Aversive Taste Memories. Neuron. 2018;100(891–9):e5.
Babic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol. 2014;722:38–47.
Travagli RA, Hermann GE, Browning KN, et al. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305.
Kushner BS, Freeman D, Sparkman J, et al. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg Obes Relat Dis. 2020;16:1505–13.
Hankir MK, Seyfried F, Miras AD, et al. Brain Feeding Circuits after Roux-en-Y Gastric Bypass. Trends Endocrinol Metab. 2018;29:218–37.
Mumphrey MB, Hao Z, Townsend RL, et al. Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit. Int J Obes (Lond). 2016;40:921–8.
Chambers AP, Wilson-Perez HE, McGrath S, et al. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303:E1076–84.
Tracy AL, Schurdak JD, Chambers JB, et al. Aversion learning can reduce meal size without taste avoidance in rats. Obesity (Silver Spring). 2016;24:606–14.
Kleinert M, Bojsen-Moller KN, Jorgensen NB, et al. Effect of bariatric surgery on plasma GDF15 in humans. Am J Physiol Endocrinol Metab. 2019;316:E615–21.
Dolo PR, Yao L, Liu PP, et al. Effect of sleeve gastrectomy on plasma growth differentiation factor-15 (GDF15) in human. Am J Surg. 2020;220:725–30.
Vila G, Riedl M, Anderwald C, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57:309–16.
Salman A, Shaaban HE, Salman M, et al. Changes in Plasma Growth Differentiation Factor-15 After Laparoscopic Sleeve Gastrectomy in Morbidly Obese Patients: A Prospective Study. J Inflamm Res. 2021;14:1365–73.
Martinussen C, Svane MS, Bojsen-Moller KN, et al. Plasma GDF15 levels are similar between subjects after bariatric surgery and matched controls and are unaffected by meals. Am J Physiol Endocrinol Metab. 2021;321:E443–52.
Luan HH, Wang A, Hilliard BK, et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell. 2019;178(1231–44):e11.
Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–40.
Borner T, Arnold M, Ruud J, Breit SN, Langhans W, Lutz TA, et al. Anorexia-cachexia syndrome in hepatoma tumour-bearing rats requires the area postrema but not vagal afferents and is paralleled by increased MIC-1/GDF15. J Cachexia Sarcopenia Muscle. 2017;8(3):417–27. https://doi.org/10.1002/jcsm.12169.
Tsai VWW, Husaini Y, Sainsbury A, et al. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018;28:353–68.
Welsh JB, Sapinoso LM, Kern SG, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–5.
Bauskin AR, Brown DA, Kuffner T, et al. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–6.
Brown DA, Ward RL, Buckhaults P, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–50.
Altena R, Fehrmann RS, Boer H. Growth differentiation factor 15 (GDF-15) plasma levels increase during bleomycin- and cisplatin-based treatment of testicular cancer patients and relate to endothelial damage. PLoS One. 2015;10:e0115372.
Hsu JY, Crawley S, Chen M, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255–9.
Breen DM, Kim H, Bennett D, et al. GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 2020;32(938–50):e6.
Petry CJ, Ong KK, Burling KA, et al. Associations of vomiting and antiemetic use in pregnancy with levels of circulating GDF15 early in the second trimester: A nested case-control study. Wellcome Open Res. 2018;3:123.
Yang L, Chang CC, Sun Z, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23:1158–66.
Emmerson PJ, Wang F, Du Y, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23:1215–9.
Mullican SE, Lin-Schmidt X, Chin CN, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23:1150–7.
Tsai VW, Zhang HP, Manandhar R, et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int J Obes (Lond). 2018;42:561–71.
Borner T, Wald HS, Ghidewon MY, et al. GDF15 Induces an Aversive Visceral Malaise State that Drives Anorexia and Weight Loss. Cell Rep. 2020;31:107543.
Borner T, Shaulson ED, Ghidewon MY, et al. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020;31(351–62):e5.
Patel S, Alvarez-Guaita A, Melvin A, et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019;29(707–18):e8.
Sabatini PV, Frikke-Schmidt H, Arthurs J, Gordian D, Patel A, Rupp AC, et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc Natl Acad Sci U S A. 2021;118(8):e2021357118. https://doi.org/10.1073/pnas.2021357118.
Frikke-Schmidt H, Hultman K, Galaske JW, et al. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol Metab. 2019;21:13–21.
Dolo PR, Widjaja J, Yao L, et al. The effect of bariatric surgery on the expression of Growth Differentiation Factor-15/Macrophage-Inhibitory Cytokine-1 (GDF-15/MIC-1) in rat. Surg Endosc. 2022;36:6205–13.
Macia L, Tsai VW, Nguyen AD, et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One. 2012;7:e34868.
Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578:444–8.
Holsen LM, Davidson P, Cerit H, et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes (Lond). 2018;42:785–93.
Joseph RJ, Alonso-Alonso M, Bond DS, et al. The neurocognitive connection between physical activity and eating behaviour. Obes Rev. 2011;12:800–12.
Lin Z, Qu S. Legend of Weight Loss: a Crosstalk Between the Bariatric Surgery and the Brain. Obes Surg. 2020;30:1988–2002.
Baboumian S, Pantazatos SP, Kothari S, et al. Functional Magnetic Resonance Imaging (fMRI) of Neural Responses to Visual and Auditory Food Stimuli Pre and Post Roux-en-Y Gastric Bypass (RYGB) and Sleeve Gastrectomy (SG). Neuroscience. 2019;409:290–8.
Bruce JM, Hancock L, Bruce A, et al. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis. 2012;8:602–8.
Li G, Ji G, Hu Y, et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology. 2019;100:229–36.
Mantione M, van de Brink W, Schuurman PR, et al. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218 (discussion E).
Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013;119:56–63.
Harat M, Rudas M, Zielinski P, et al. Nucleus accumbens stimulation in pathological obesity. Neurol Neurochir Pol. 2016;50:207–10.
Casquero-Veiga M, Garcia-Garcia D, Desco M, et al. Understanding Deep Brain Stimulation: In Vivo Metabolic Consequences of the Electrode Insertional Effect. Biomed Res Int. 2018;2018:8560232.
Contreras Lopez WO, Navarro PA, Crispin S. Effectiveness of Deep Brain Stimulation in Reducing Body Mass Index and Weight: A Systematic Review. Stereotact Funct Neurosurg. 2022;100:75–85.
Shivacharan RS, Rolle CE, Barbosa DAN, et al. Pilot study of responsive nucleus accumbens deep brain stimulation for loss-of-control eating. Nat Med. 2022;28:1791–6.
Mahajan UV, Ojukwu DI, Azagury DE, et al. Can responsive deep brain stimulation be a cost-effective treatment for severe obesity? Obesity (Silver Spring). 2022;30:338–46.
Kim SH, Chung JH, Kim TH, et al. The effects of repetitive transcranial magnetic stimulation on eating behaviors and body weight in obesity: A randomized controlled study. Brain Stimul. 2018;11:528–35.
Devoto F, Ferrulli A, Zapparoli L, et al. Repetitive deep TMS for the reduction of body weight: Bimodal effect on the functional brain connectivity in “diabesity.” Nutr Metab Cardiovasc Dis. 2021;31:1860–70.
Encarnacion M, Dampil OA, Damian L, et al. Efficacy of Repetitive Transcranial Magnetic Stimulation (rTMS)in Inducing Weight Loss among Obese Filipino Patients: A Randomized Controlled Trial. J ASEAN Fed Endocr Soc. 2020;35:181–9.
Mostafavi SA, Khaleghi A, Mohammadi MR, et al. Is transcranial direct current stimulation an effective modality in reducing food craving? A systematic review and meta-analysis. Nutr Neurosci. 2020;23:55–67.
Stinson EJ, Travis KT, Magerowski G, et al. Improved food Go/No-Go scores after transcranial direct current stimulation (tDCS) to prefrontal cortex in a randomized trial. Obesity (Silver Spring). 2022;30:2005–13.
de Araujo C, Fitz RC, Natividade GR, et al. The effect of transcranial direct current stimulation along with a hypocaloric diet on weight loss in excessive weight people: A pilot randomized clinical trial. Clin Nutr ESPEN. 2020;40:68–76.
Ljubisavljevic M, Maxood K, Bjekic J, et al. Long-Term Effects of Repeated Prefrontal Cortex Transcranial Direct Current Stimulation (tDCS) on Food Craving in Normal and Overweight Young Adults. Brain Stimul. 2016;9:826–33.
Enriquez-Geppert S, Huster RJ, Herrmann CS. EEG-Neurofeedback as a Tool to Modulate Cognition and Behavior: A Review Tutorial. Front Hum Neurosci. 2017;11:51.
Al-Hiyali MI, Ishak AJ, Harun H, et al. Stimulation The Prefrontal Cortex By EEG-Neurofeedback Training In High Body Mass Index Individuals. 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES)2018; 189–93.
Kohl SH, Veit R, Spetter MS, et al. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609.
Burneo JG, Faught E, Knowlton R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463–4.
Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond). 2007;31:1756–9.
Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915–22.
Apovian CM, Shah SN, Wolfe BM, et al. Two-Year Outcomes of Vagal Nerve Blocking (vBloc) for the Treatment of Obesity in the ReCharge Trial. Obes Surg. 2017;27:169–76.
Cracchiolo M, Ottaviani MM, Panarese A, Strauss I, Vallone F, Mazzoni A, et al. Bioelectronic medicine for the autonomic nervous system: clinical applications and perspectives. J Neural Eng. 2021;18(4). https://doi.org/10.1088/1741-2552/abe6b9.
Li S, Kim Y, Chen JDZ, et al. Intestinal Electrical Stimulation Alters Hypothalamic Expression of Oxytocin and Orexin and Ameliorates Diet-Induced Obesity in Rats. Obes Surg. 2021;31:1664–72.
Dong Y, Zhang Y, Li S, et al. Intestinal Electrical Stimulation Enhances Release of Postprandial Incretin Hormones Via Cholinergic Mechanisms. Obes Surg. 2021;31:1957–66.
Aberle J, Busch P, Veigel J, et al. Duodenal Electric Stimulation: Results of a First-in-Man Study. Obes Surg. 2016;26:369–75.
Chaudhari SN, Harris DA, Aliakbarian H, et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol. 2021;17:20–9.
Ding L, Zhang E, Yang Q, Jin L, Sousa KM, Dong B, et al. Vertical sleeve gastrectomy confers metabolic improvements by reducing intestinal bile acids and lipid absorption in mice. Proc Natl Acad Sci U S A. 2021;118(6):e2019388118. https://doi.org/10.1073/pnas.2019388118.
Troseid M, Nestvold TK, Rudi K, et al. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36:3627–32.
Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–93.
Kwon IG, Kang CW, Park JP, et al. Serum glucose excretion after Roux-en-Y gastric bypass: a potential target for diabetes treatment. Gut. 2020.
Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2.
Acknowledgements
The authors are grateful to Professor Marco Bueter, Head of the Bariatric Surgery Program at the University Hospital Zurich, for his critical review of the manuscript.
Funding
Open access funding provided by University of Zurich. This research did not receive any specific grant or funding from agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
Study design: all authors.
Drafting the “Introduction” and “Future Outlook”: DG, LDF.
Drafting the section on “Intestinal Nutrient Sensing”: EM.
Drafting the section on “Intestinal Milieu”: MKH.
Drafting the section on “Nutrient Sensing Via the Vagus Nerve”: LDF.
Drafting the section on “The Role of Hindbrain”: TB.
Drafting the section on “Weight Loss by Neurostimulation”: BF.
Critical revision and final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Informed Consent
Does not apply.
Human and Animal Rights
Does not apply.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Bariatric surgery enhances intestinal nutrient sensing by increased hormonal (between-meal satiety) and vagal (intra-meal satiation) signaling.
• Postbariatric changes in the intestinal milieu (especially microbiota and bile acids) stabilize the intestinal epithelial barrier and decrease systemic inflammation.
• Cytokine growth differentiation factor 15 (GDF15) has a potent food intake and body weight suppressive effect by acting on hindbrain centers of nausea, and its level is increased after bariatric surgery.
• Superior weight loss can be achieved in patients with obesity by stimulation of the vagal nerve compared to cortical electric manipulation of the homeostatic brain centers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frick, L.D., Hankir, M.K., Borner, T. et al. Novel Insights into the Physiology of Nutrient Sensing and Gut-Brain Communication in Surgical and Experimental Obesity Therapy. OBES SURG 33, 2906–2916 (2023). https://doi.org/10.1007/s11695-023-06739-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06739-4