Abstract
Background
The combination of pneumoperitoneum and intraoperative retraction of the left lobe of the liver leads to hepatocellular injury during laparoscopic gastric surgery. Fatty livers are more susceptible to ischaemic insults. This trial investigated whether the antioxidant N-acetylcysteine (NAC) reduced liver injury during laparoscopic sleeve gastrectomy (LSG).
Methods
Patients undergoing LSG were randomised (single blinded) to receive intraoperative NAC infusion or standard anaesthetic treatment. Blood samples were taken before and after surgery (days 0 to 4). Primary endpoints included serum aminotransferases. Secondary measures were C-reactive protein, weight cell count (WCC), cytokines (interleukin 6 and 10) and cytokeratin-18 as markers of apoptosis. Intraoperative liver biopsy samples were assessed using a locally developed injury score.
Results
Twenty patients (14 females, mean age 44.5 (SEM ± 2.9) years, mean BMI 60.8 (SEM ± 2.4) kg/m2) were recruited (NAC n = 10, control n = 10). The trial was stopped early after a planned interim analysis. Baseline liver function was similar. The peak rise in liver enzymes was on day 1, but levels were not significantly different between the groups. Rates of complications and length of stay were not significantly different. Secondary outcome measures, including white cell count (WCC), cytokines and cytokeratin (CK)-18 fragments, were not different between groups. Liver injury scores did not differ significantly.
Conclusions
NAC did not reduce intraoperative liver injury in this small number of patients. The heterogenous nature of the study population, with differences in co-morbidities, body mass index and intraabdominal anatomy, leads to a varied post-operative inflammatory response. Significant hepatocyte injury occurs through both necrosis and apoptosis.
Similar content being viewed by others

Introduction
Clinically significant post-operative complications occur in approximately 10 % of bariatric surgical patients, increasing hospital stay [1]. Up to 18 % of patients with pre-existing liver disease have progressive hepatic dysfunction following bariatric surgery, with severity ranging from transient elevation of liver transaminases [2] to organ dysfunction [3] and rarely liver failure causing death [4]. Although bariatric surgery leads to long-term reduction in liver steatosis in a majority of patients, inflammatory changes and fibrosis scores either increase or are unchanged in at least 35 % of patients [5]. Progression of fatty liver disease to steatohepatitis and fibrosis occurs due to oxidative stress and chronic inflammation [6]. Steatotic hepatocytes are more vulnerable to toxic insults and hypoxia, being less able to tolerate the accompanying mitochondrial dysfunction and buffer the increased oxidative stress. Thus, potential antioxidant and anti- inflammatory effects of NAC are an attractive treatment for patients with NAFLD [7].
During laparoscopic bariatric surgery, the left lobe of the liver is retracted to expose the stomach, causing ischaemia. Release of the retractor at the end of surgery can cause ischaemia-reperfusion injury. Following gastric bypass, a 6-fold rise in aspartate transaminase and alanine transaminase from baseline levels, causing a liver injury that may contribute to post-operative inflammatory response [2, 8]. Although usually self-limiting, a larger post-operative inflammatory response may predispose the patient to a worse response to further insults, such as anastomotic leakage or post-operative infective—a so-called “second hit” [9].
Non-alcoholic fatty liver disease (NAFLD) has a number of structural and metabolic differences to non-steatotic liver [10] and are more likely to die, more readily through necrosis than apoptosis [11]. As a consequence, tolerance to ischaemia during bariatric surgery is reduced. The common inflammatory processes implicated in the pathogenesis of insulin resistance, obesity and fatty liver disease are also of great relevance to the pathological effects of ischaemia-reperfusion injury (IRI), especially leptin, adiponectin and resistin [12, 13].
A number of animal models of IRI have shown beneficial effects of NAC, including increased bile flow, improved sinusoidal blood flow, decreased post-operative liver transaminases and reduced evidence of injury on liver histopathology [14] In nine clinical trials in human orthotopic liver transplantation, NAC was administered either to donors before organ retrieval or to recipients before graft revascularisation. Three showed a significant reduction in post-operative liver enzymes, although the clinical significance in terms of complications and graft survival could not be evaluated in these small studies [15]. In an RCT of NAC in liver resection, Robinson et al. found no differences in clinical outcomes between groups; though on day 3, alanine transaminase (ALT) levels were lower in the treatment group [16].
The aim of this study was to assess the effect of bariatric surgery upon hepatocellular injury and the impact of the administration of NAC upon this. Amelioration of the unavoidable post-operative inflammatory response may reduce the subsequent impact of other complications. The study hypothesis was that NAC infusion would reduce hepatocellular injury and associated inflammatory response.
Methods
Trial Design and Recruitment
This study was approved by the Local Research Ethics Committee and the Medicines and Healthcare-related products Regulatory Agency of the United Kingdom (UK). Each patient gave fully informed, written consent prior to enrolment into this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patients were recruited from the obesity clinic at Kings College Hospital and randomised on the day of surgery into a treatment group (NAC group) or control group, receiving standard care.
Inclusion criteria were patients aged 18 to 75 years inclusive, meeting the published National Institute of Clinical Excellence indications for weight loss surgery, that is they must have BMI >40 or >35 kg/m2 with obesity-related complications and are undergoing laparoscopic sleeve gastrectomy [17]. Patients were excluded if they had a history of chronic liver disease, abnormal hepatitis serology or autoantibody screen, previous liver surgery, a history of active psychiatric illness, a known bleeding tendency or prescribed anticoagulant medications or a known allergy to NAC or related compounds.
Subjects randomised to the treatment group received N-acetyl cysteine infusion (Aurum Pharmaceuticals Ltd, Romford, UK) at a standard 150 mg/kg in 200 ml 5 % dextrose over 15 min at induction of anaesthesia followed by an infusion of 50 mg/kg in 500 ml of 5 % dextrose during surgical retraction of the liver for a maximum of 4 h, together with standard anaesthetic medications. The maximum dose was limited to 16.5 g for loading dose and 5.5 g for infusion, making a maximum total dose of 22 g per study participant. The control subjects received standard anaesthetic medications alone.
Treatment of Patients
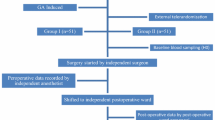
Patients were treated as per routine practice. The LSG was performed by a single experienced surgeon (AP) for all patients. Six trocars were utilised and LSG was performed with standard techniques using a 38-French bougie. A 12 mmHg carbon dioxide pneumoperitoneum was created. A liver biopsy from the left lateral section of the liver was taken using a spring-loaded core biopsy cannula. A fixed Nathanson liver retractor was placed under the left lateral section of the liver to facilitate clear visualisation of the hiatus. After devascularisation of the greater curvature using ultrasonic shears, the LSG commenced 4–6 cm proximal to the pylorus on the greater curvature and continued towards the angle of His. The completed staple line was reinforced with absorbable sutures. A further liver biopsy from the left lateral section was taken before release of pneumoperitoneum. Patients received low molecular weight heparin and wore compression stockings for thromboembolism prophylaxis.
Post-operative diet consisted of fluids only for 4 weeks, puréed consistency foods for a further 2–4 weeks, followed by soft foods for an additional 4 weeks, followed by a gradual return to normal foods in reduced portions.
Sample Collection
Serial fasting serum and plasma samples were taken before surgery, immediately following surgery and on post-operative days 1, 2, 3 and 4. Samples were sent for routine biochemistry and haematology analysis in the Department of Pathology using an automated multi-analyser. The remaining samples were stored in aliquots at −80 °C for batch analysis of other markers.
TNF(tumour necrosis factor)α, interleukin (IL)-6, IL-10 and the adipocytokines: leptin, resistin and adiponectin were measured using a bead-based multiplex array (Fluorokine® MultiAnalyte Profiling Kit (R&D Systems Europe Ltd, Abingdon, UK) and the results were read on a Luminex® Analyzer (Luminex B.V., Oosterhout, The Netherlands).
Enzyme-linked immunosorbent assay (ELISA) were used for cytokeratin (CK)-18 M65 and M30 (M65® Classic and M30 Apoptosense®, PEVIVA AB, Bromma, Sweden, supplied through BIOAXXESS® UK, Malvern, UK), TNF-related apoptosis-inducing ligand (TRAIL) and FasLigand (FasL) (Quantikine ELISA, R & D Systems Europe Ltd, Abingdon, UK). The manufacturers’ protocols were followed.
Efficacy Variables
The following patient specific data were collected as part of the study: demographic details, medical history, body mass index, operating time, time of liver retraction, blood loss, length of stay and perioperative complications.
Primary outcome variables were alanine transaminase (ALT) and aspartate aminotransferase (AST). Secondary outcome measures included WCC, platelets (PLT), C-reactive protein (CRP), inflammatory cytokines: TNFα, IL-6 and IL-10 and adipocytokines: leptin, adiponectin and resistin and markers of liver cell death: CK-18, TRAIL and FasL.
Histopathology
NAFLD Activity Scoring (NAS) was undertaken blind, according to the criteria set out by Kleiner and Brunt [18], defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2); thus ranging from 0 to 8. Scores of ≥5 are compatible with a diagnosis of steatohepatitis. Fibrosis was scored 0–4 according to the Kleiner-Brunt definitions [18].
For assessment of hepatocellular injury a local liver injury score was developed for this study, based on the non-alcoholic steatohepatitis (NASH) Clinical Research Network Scoring System Definitions set out by Kleiner et al. [18], with additional modifications to include other potentially pertinent histological changes (see Table 1).
Sample Size Calculation
In a previous study, the AST increased significantly from baseline (24 ± 6) and peaked at 24 h after laparoscopic (152 SD ± 102 U/L) and open (231 SD ± 518 U/L) RYGB but there was no significant difference in AST levels between study groups (Nguyen et al. 2003 [2]).
In order to conduct a study with 80 % power to detect a 50 % reduction in the post-operative rise in AST in relation to placebo in the population of laparoscopy patients, assuming an increase in AST level of 128 U/L and a pooled standard deviation of 100 U/L, the 50 % reduction corresponds to an effect of size 0.64, yielding a sample size of 40 patients in each of the two groups. Interim analyses were planned after 2 years. Clinical outcomes were monitored by the principal investigator and the co-investigators and overseen by the institution’s clinical trials monitoring team and the Medicines and Healthcare-related products Regulatory Authority (UK).
Randomisation and Blinding
A computer-generated randomisation sequence was used, in blocks of 10, in a 1:1 ratio. Allocation concealment was performed using sequential numbered sealed envelopes, produced by an independent statistician. Randomisation took place on the morning of surgery. Participants were blinded to treatment allocation. The clinical team was not blinded, as there was no placebo infusion used. Biomarker laboratory analysis was performed blinded to treatment group.
Statistical Analysis
Statistical analyses were performed using SPSS 20 (SPSS, USA). Demographic data and intraoperative variables were compared between groups using independent samples t test and Fisher’s exact test. Complications between groups were compared using Pearson’s chi-square test. A secondary analysis of changes in parameters over time within groups was performed using repeated measures ANOVA. Post hoc comparisons between different time points were performed after applying a Bonferroni correction. Total area under curve (AUC) for each parameter was also calculated to give a more general measure of post-operative change.
To assess the interaction between demographic and intraoperative factors and the primary outcome measures, correlations were performed using Pearson’s correlation, including log10 transformation for non-parametric variables. Clinical significance was determined if p < 0.05.
Results
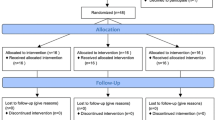
Twenty patients participated in this study from July 2009 to August 2012 (see Fig. 1 for CONSORT diagram) [19]. The study was stopped after a planned interim analysis of 20 patients. Both clinical and laboratory markers displayed a wide variation in data, with large confidence intervals and standard deviations. Such variance was likely to be due to multiple factors but included deficiencies in study design. It was noted that large differences in the timing of liver retraction and its apparent effect on the liver function were also evident. Taken together with the slow rate of recruitment, the investigators felt that it was unlikely that continuation of the study to full recruitment was impractical and unlikely to reach a more reliable and valid conclusion. Therefore, the study was terminated.
Flow diagram of patients through the trial (according to the Consolidated Standards of Reporting Trials Statement) [19]
The baseline characteristics, including age, BMI and co-morbidities were similar in both groups. All patients had minimal regular alcohol intake, with one patient having given up heavy alcohol intake more than a decade previously. Only one patient was a smoker. Operating times, liver retraction times and recorded estimated blood loss were all similar. A summary of the characteristics of the study population and the intraoperative parameters is given in Table 2.
Adverse Events
Of 20 patients, 10 patients had significant complications, meeting criteria for serious adverse events, including two patients with complications of Clavien-Dindo grade 3 or worse [20]. One patient in the treatment group had an increase in airway pressure, on induction of anaesthesia, leading to abandonment of surgery. There were no sequelae and only the demographic details are included in this analysis, as no samples were taken.
One patient had a post-operative haemorrhage, then a staple line leakage requiring two further surgeries, one patient required a two-unit blood transfusion post-operatively and one patient with pre-existing obesity hypoventilation syndrome required non-invasive ventilator support for post-operative respiratory difficulties. Four patients were readmitted within 30 days of discharge. Complications are summarised in Tables 3 and 4.
Primary Outcome Measures
There was a rise in ALT and AST at the end of surgery and on post-operative day 1 (p < 0.001) but no difference between treatment groups was detected (Fig. 2). Wide variations in post-operative transaminase rises between subjects in both groups were found.
Secondary Outcome Measures
There were no significant differences between treatment groups for changes in AST, WCC, CRP and platelets (see Fig. 3). There was a significant change in WCC between baseline, end of surgery and day 1 (p < 0.001). CRP lagged behind and rose significantly on days 1 and 2 (p < 0.001), when it reached a plateau, before falling off more slowly. Platelet count was depressed on day 1 and day 2 from baseline (p < 0.01).
Liver Histopathology Scores
The majority of patients had evidence of significant liver pathology, including 11 patients with NASH. Assessment of liver histology showed no significant differences between treatment groups, with only 5/19 patients having minimal signs of steatosis.
The mean difference in liver injury score between treatment groups of the start and end of surgery injury scores were not significantly different (mean difference in scores control 0.50 ± SEM 0.52 versus NAC 1.56 ± 0.58, p = 0.19).
Plasma Cytokines
There are large changes in cytokines following surgery (Fig. 4), with peak changes occurring within an hour of surgery in IL-6 and IL-10 (p < 0.01). TNFα levels are not significantly changed after surgery until day 2, when they fall to <50 % of baseline (p = 0.001). Changes in adipocytokines are more difficult to interpret and there are no significant differences within 24 h of surgery or between treatment groups (see Figs. 5 and 6).
Cytokeratin-18 Fragments
There is a large rise in CK-18 post-operatively, indicating significant cellular damage but there were no significant differences between treatment groups. Both necrosis and apoptosis occurs. TRAIL and FasL were not affected by NAC infusion and these data suggest they may not play a significant role in the cellular damage that occurs in this scenario.
Correlations
NAS score at the start of operation strongly correlated with the AUC of ALT and AST (**p = 0.003 and p = 0.008 respectively). Blood loss and time of liver retraction correlated with WCC and CRP (*p < 0.05). Operation time was strongly associated with longer liver retraction times and greater blood loss (p < 0.001). Operation time correlated strongly with ALT 24 h fold change (Pearson’s r = 0.556, p = 0.017).
Initial increase in M30 fragments correlates strongly with ALT 24 h fold-change (r = 0.578, p = 0.024) and with BMI (r = 0.613, p = 0.012). There are no correlations with TRAIL and FasL levels.
Discussion
The Effect of NAC on Liver Function and Histology
During laparoscopic bariatric surgery, the combination of pneumoperitoneum and mechanical liver retraction causes hepatocellular injury. This study shows that by the end of surgery, a 5-fold increase in ALT occurs. The injury peaks by the first post-operative day. The elevation is associated with a concomitant elevation in WCC and CRP.
This is the first report of the use of NAC or any other antioxidant as a method of attenuating intraoperative liver injury in the morbidly obese. The rationale for this study was based on evidence in our unit that NAC leads to improvement in measures of quality and metabolic function of hepatocytes isolated from steatotic livers [21]. The administration of NAC in the perioperative period did not have a significant effect on post-operative parameters. There were no significant differences between treatment groups in ALT, AST, WCC, CRP and platelet counts.
The locally developed liver injury score was used to demonstrate that histological changes did occur during surgery, and the change in injury scores due to surgery was statistically significant, although the rise in score was only on average 1 point. There are no other reports of serial intraoperative liver biopsies in humans. Although the left liver lobe looks discoloured and bruised after the removal of the liver retractor, these results demonstrate that histopathological changes at that point are limited. These data suggest that histological changes due to pneumoperitoneum are subtle. Given that markers of liver injury and inflammation continue to rise on post-operative days 1 and 2, it is likely that further histopathological changes may become discernible later. It would neither be safe or ethical to obtain liver tissue after the patient leaves the operating theatre in a clinical setting. Therefore, circulating markers of liver injury are more useful in this context.
NAS score correlated strongly with the AUC of ALT and AST, confirming that patients with higher grades of NAFLD/NASH are vulnerable to more hepatocellular damage [10, 22]. Operating and liver retraction times did not directly correlate with ALT or AST levels but a correlation between log10 transformed operating time and the 24 h ALT fold change was noted, in keeping with other studies [23, 24]. Liver retraction time correlated with AUC-CRP, indicating that inflammatory response is associated with liver injury.
Clinical Outcomes
The study groups were well matched, with similar demographic parameters. There was a high incidence of other obesity-related complications, especially diabetes, hypertension, hypercholesterolemia and obstructive sleep apnoea. Operating times, intraoperative blood loss and baseline outcome measures were similar between the groups.
The trial was not powered to detect differences in clinical outcomes. BMI >60 kg/m2 is associated with higher risk of complications following bariatric surgery [25]. The rate of life-threatening complications was low, although there were four readmissions and one patient with staple line leakage. Infusion of NAC was potentially associated with one adverse event of increased airway pressure, leading to termination of anaesthesia and abandonment of surgery.
The Effect of NAC on Cytokines
IL-6 levels peaked by the end of surgery and began to return to baseline on post-operative day 1. Bariatric surgery is associated with significant falls in IL-6 over 6–24 months [26]. IL-6 rises after any surgery but the magnitude of rise after laparoscopic surgery is smaller [27]. In turn, it stimulates production of CRP by the liver as part of the inflammatory response [28]. This study confirms this relationship, as 24 h fold change of IL-6 correlated strongly with peak CRP fold change from baseline (p = 0.016).
IL-10 underwent a mean 193-fold change after surgery (95% CI 37–350). This dramatic increase may reflect the fact that most of the patients were super-obese (BMI > 60 kg/m2), diabetic and had NASH—all factors associated with elevated IL-10 [29, 30]. IL-10 has traditionally been viewed as an anti-inflammatory cytokine [31].
Changes in adipocytokines, leptin, resistin and adiponectin were not significant over the first two post-operative days and do not support the existing literature suggesting that these hormones play an important role in the acute phase response [32]. Patients with NAFLD have evidence of chronic low-grade inflammation and altered innate immunity which contributes to progression of their liver disease [33]. Post-surgical stress and metabolic changes may affect the liver [34]. At present these mechanisms are poorly understood and modulation of the immune response by targeting specific pro-inflammatory cytokines has had little clinical success [35].
Apoptosis Markers
There is only one longitudinal study showing that CK-18 fragment levels fall 6 months after surgery in patients with NASH [36]. Our data is the first of its kind measuring CK-18 fragments in the first 4 days after bariatric surgery. It shows that proportional levels of apoptosis do not increase significantly after surgery. Similar to the elevation in transaminases, this study shows that both M30 and M65 fragment concentrations increase dramatically on the first post-operative day, before returning to near baseline by day 4.
Limitations of Study
The interpretation of the results are hampered by the small sample size and premature closure of the trial.
The major drawback of the study was a lack of a standardised, reproducible toxic insult to the liver during intraoperative liver retraction with the Nathanson liver retractor. The pressure applied to liver varied from patient to patient depending on their body habitus, intraabdominal dimensions and size and texture of the liver. There are presently no routine clinical methods of measuring tissue oxygen tension or pressure within the liver. This lack of uniformity lead the investigators to conclude that a major study redesign would be required to reach an robust conclusion, even with full recruitment to the planned total sample size of 80.
Patients were not stratified by their other co-morbidities and allowed to continue their regular medications. Eleven (58 %) of patients completing the study were taking cholesterol-lowering medications, including various statins and fenofibrate. These drugs are known to cause liver dysfunction [37], although it should be noted that all patients had normal liver function at baseline.
In this study, the timing of the injury to the liver was intraoperative. Taking the results presented here which show peak transaminase rise on post-operative day 1 together with other evidence from the ischaemia-reperfusion injury literature, it is likely that the effects of liver retraction, due to compression and ischaemia, might persist for up to 12 and perhaps more than 24 h after surgery [38]. It could be argued that a one-off infusion of NAC is insufficient to counteract this injury. Many animal models investigating NAC and IRI have used a continuous infusion lasting for greater than 6 hours [39]. In effect, the methods use herein describe an in vivo model of hepatocellular injury in fatty (and indeed non-fatty) liver, and a similar methodology may be used to test other potential therapeutic interventions.
Conclusions
This study shows that bariatric surgery results in ischaemic-reperfusion injury to the liver. This can be measured with biochemical markers and histopathological scoring systems. N-acetylcysteine did not affect this outcome.
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA: J Am Med Assoc. 2004;292:1724–37.
Nguyen NT, Braley S, Fleming NW, et al. Comparison of postoperative hepatic function after laparoscopic versus open gastric bypass. Am J Surg. 2003;186:40–4.
Brolin RE, Bradley LJ, Taliwal RV. Unsuspected cirrhosis discovered during elective obesity operations. Arch Surg. 1998;133:84–8.
Cotler SJ, Vitello JM, Guzman G, et al. Hepatic decompensation after gastric bypass surgery for severe obesity. Dig Dis Sci. 2004;49:1563–8.
Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol: Off Clin Prac J Am Gastroenterol Assoc. 2008;6:1396–402.
Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210–5.
Mehta K, Van Thiel DH, Shah N, et al. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289–93.
Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–66.
Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: role of oxidants as priming agents. Antioxid Redox Signal. 2002;4:69–83.
Tashiro H, Kuroda S, Mikuriya Y, Ohdan H (2013) Ischemia-reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surgery Today
Selzner M, Rudiger HA, Sindram D, et al. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–8.
Whitehead JP, Richards AA, Hickman IJ, et al. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes, Obes Metab. 2006;8:264–80.
Jimenez-Castro MB, Casillas-Ramirez A, Mendes-Braz M, et al. Adiponectin and resistin protect steatotic livers undergoing transplantation. J Hepatol. 2013;59:1208–14.
McKay A, Cassidy D, Sutherland F, et al. Clinical results of N-acetylcysteine after major hepatic surgery: a review. J Hepato-Biliary-Pancreat Surg. 2008;15:473–8.
Jegatheeswaran S, Siriwardena AK. Experimental and clinical evidence for modification of hepatic ischaemia-reperfusion injury by N-acetylcysteine during major liver surgery. HPB: Off J Int Hepato Pancreato Biliary Assoc. 2011;13:71–8.
Robinson SM, Saif R, Sen G, et al. N-acetylcysteine administration does not improve patient outcome after liver resection. HPB: Off J Int Hepato Pancreato Biliary Assoc. 2013;15:457–62.
Owen-Smith A, Kipping R, Donovan J, et al. A NICE example? Variation in provision of bariatric surgery in England. BMJ. 2013;346:f2453.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Sagias FG, Mitry RR, Hughes RD, et al. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010;19:1487–92.
Vetelainen R, van Vliet A, Gouma DJ, et al. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30.
Sammour T, Mittal A, Loveday BP, et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg. 2009;96:836–50.
Nguyen NT, Goldman CD, Ho HS, et al. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194:557–66. discussion 566–557.
Sanni A, Perez S, Medbery R, et al. Postoperative complications in bariatric surgery using age and BMI stratification: a study using ACS-NSQIP data. Surg Endosc. 2014;28:3302–9.
Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res: Off J Eur Histamine Res Soc. 2012;61:789–807.
Jacobi CA, Wenger F, Opitz I, et al. Immunologic changes during minimally invasive surgery. Dig Surg. 2002;19:459–63.
Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3.
Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94:450–8.
Rabelo F, Oliveira CP, Faintuch J, et al. Pro- and anti-inflammatory cytokines in steatosis and steatohepatitis. Obes Surg. 2010;20:906–12.
Dali-Youcef N, Mecili M, Ricci R, et al. Metabolic inflammation: connecting obesity and insulin resistance. Ann Med. 2013;45:242–53.
Behnes M, Brueckmann M, Lang S, et al. Alterations of adiponectin in the course of inflammation and severe sepsis. Shock. 2012;38:243–8.
Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;3:377–85.
Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–8.
Hutchins NA, Unsinger J, Hotchkiss RS, et al. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–33.
Kahraman A, Sowa JP, Schlattjan M, et al. Fetuin-A mRNA expression is elevated in NASH compared with NAFL patients. Clin Sci. 2013;125:391–400.
Geng Q, Ren J, Chen H, et al. Adverse events following statin-fenofibrate therapy versus statin alone: a meta-analysis of randomized controlled trials. Clin Exp Pharmacol Physiol. 2013;40:219–26.
Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, et al. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–9.
Fusai G, Glantzounis GK, Hafez T, et al. N-acetylcysteine ameliorates the late phase of liver ischaemia/reperfusion injury in the rabbit with hepatic steatosis. Clin Sci. 2005;109:465–73.
Acknowledgments
The authors would like to thank Hannah Mason and Ingrid Brumarescu of the Joint Clinical Trials Office for their meticulous assistance in ensuring the conduct of this trial met the standards set by the MHRA(UK). We are very grateful to Joanna Riddoch-Contreras and Abdul Hye of the Institute of Psychiatry for their help with the Luminex analysis. We would also like to thank Cheryl Goulias, Yvonne Connolly and Beth Murgatroyd for their generous logistical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Ragai R. Mitry and Ameet G. Patel are joint senior authors.
Rights and permissions
About this article
Cite this article
Belgaumkar, A.P., Carswell, K.A., Hughes, R.D. et al. The Effect of Intraoperative N-Acetylcysteine on Hepatocellular Injury During Laparoscopic Bariatric Surgery. A Randomised Controlled Trial. OBES SURG 26, 1254–1265 (2016). https://doi.org/10.1007/s11695-015-1904-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1904-3