Abstract
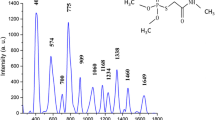
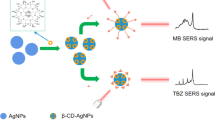
Pyraclostrobin (methyl N-(2-{[1-(4-chlorophenyl)-1 H-pyrazol-3-yl]oxymethyl}phenyl) N-methoxycarbamate) is a synthetic fungicide used against a wide range of plant pathogens. Its mode of action/mechanisms of toxicity is the inhibition of mitochondrial respiration of fungi. Given the extended application of pyraclostrobin in the agricultural sector, and its potential hazard for humans, it is necessary to monitor its residual traces in agricultural products to ensure food safety. This work reports the first application of surface-enhanced Raman spectroscopy (SERS) for qualitative and semi-quantitative detections of the fungicide both in standard solutions and in lemon peel extractions from contaminated samples at laboratory scale. For this, a simple, low-cost and easy-to-construct SERS active substrate consisting of a ring of silver nanoparticles (AgNPs) immobilized on a glass slide was used. Pyraclostrobin up to 6 × 10− 5 M concentration on the lemon peel was detected in a fast and neat procedure. The adsorption mechanism of the pesticide on the AgNPs was characterized by comparison between the normal Raman and SERS spectra of the substance and by quantum-chemical calculations of different pyraclostrobin-Ag3 complexes. Theoretical predictions and experimental data suggest that the molecule is able to adopt different orientations on the substrate.







Similar content being viewed by others
References
E. Ammermann, G. Lorenz, K. Schelberger, B. Mueller, R. Kirstgen, H. Sauter, BAS 500 F: the new broad spectrum strobilurin fungicide, in: Brighton Crop Protection Conference, Pests and Diseases, British Crop Protection Council, UK, 541–548 (2000)
D.W. Bartlett, J.M. Clough, C.R.A. Godfrey, J.R. Godwin, A.A. Hall, S.P. Heaney, S.J. Maund, Pestic. Outlook. 12, 143–148 (2001). https://doi.org/10.1039/B106300F
D.W. Bartlett, J.M. Clough, J.R. Godwin, A.A. Hall, M. Hamer, B. Parr-Dobrzanski, Pest. Manag. Sci. 58, 649–662 (2002). https://doi.org/10.1002/ps.813
D. Fernández-ortuño, J.A. Torés, A. De Vicente, A. Pérez-garcía, in Fungicides (2010), ed. By O. Carisse (InTech, Croatia, 2010), p. 203. Available from: http://www.intechopen.com/books/fungicides/the-qoifungicides-the-rise-and-fall-of-a-successful-class-of-agricultural-fungicides
World Health Organization, Food and Agriculture Organization of the United Nations, Pesticide residues in food: 2018: toxicological evaluations / Joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group, on Pesticide Residues, Berlin, Germany, 18–27 September 2018 (World Health Organization, 2019), https://apps.who.int/iris/handle/10665/325787. Accessed 11 July 2023
(Environmental Protection Agency (EPA), Pyraclostrobin; Pesticide Tolerances, Environmental Protection, Agency, 2010), https://www.federalregister.gov/documents/2010/07/21/2010-17793/pyraclostrobin-pesticide-tolerances. Accessed 11 July 2023
A. Çayır, M. Coskun, M. Coskun, Environ. Toxicol. 29, 723–732 (2012)
H. Cobanoglu, B. Coskun, A. Çayir, Pestic. Phytomed. 34, 61–67 (2019). https://doi.org/10.2298/PIF1901061C
A. Dhawan, D. Anderson, The Comet Assay in Toxicology, vol. 5 (Royal Society of Chemistry, London, UK, 2009)
F.A. Esteve-Turrillas, J.V. Mercader, C. Agulló, A. Abad-Somovilla, A. Abad-Fuentes, J. Chromatogr. A 1218(30), 4902–4909 (2011). https://doi.org/10.1016/j.chroma.2011.03.022
F. Zhang, L. Wang, L. Zhou, D. Wu, H. Pan, C. Pan, Ecotox Environ. Safe. 78, 116–122 (2012). https://doi.org/10.1016/j.ecoenv.2011.11.003
X. Guo, W. Wu, N. Song, J. Li, D. Kong, X. Kong, J. He, K. Chen, Z. Shan, Hum. Ecol. Risk Assess: An. International Journal. 23(1), 67–81 (2017). https://doi.org/10.1080/10807039.2016.1222579
X. Fan, S. Zhao, X. Chen, J. Hu, Food Anal. Methods. 11(5), 1312–1320 (2018). https://doi.org/10.1007/s12161-017-1065-1
X. Liu, Y. Yang, Y. Chen, Q. Zhang, P. Lu, D. Hu, Food Additives & Contaminants: Part A (2019) https://doi.org/10.1080/19440049.2019.1640894
Y. Liu, S. Jiao, Y. Chang, X. Lu, P. Liu, Y. Zhao, C. Zha, L. Shen, Y. Guo, G. Zhu, Food Agric. Immunol. 31(1), 985–1003 (2020). https://doi.org/10.1080/09540105.2020.1797640
L. Lv, Y. Su, B. Dong, W. Lu, J. Hu, X. Liu, Molecules. 27(14), 4410 (2022). https://doi.org/10.3390/molecules27144410
L. Guerrini, D. Graham, Chem. Soc. Rev. 41, 7085–7107 (2012)
V. Giannini, A.I. Fernandez-Dominguez, Y. Sonnefraud, T. Roschuk, R. Fernandez-Garcia, S.A. Maier, Small. 6, 2498–2507 (2010)
J. Chen, M. Huang, L. Kong, M. Lin, Carbohydr. Polym. 205, 596–600 (2019). https://doi.org/10.1016/j.carbpol.2018.10.059
L. Jiang, K. Gu, R. Liu, S. Jin, H. Wang, C. Pan, SN Appl. Sci. 1, 627 (2019). https://doi.org/10.1007/s42452-019-0619-9
H. Zhou, X. Li, L. Wang, Y. Liang, A. Jialading, Z. Wang, J. Zhang, Rev Anal Chem. 40(1), 173–186 (2021) https://doi.org/10.1515/revac-2021-0132
L. Yande, Z. Yuxiang, W. Haiyang, Y. Bing, Int. J. Agric. & Biol. Eng. 9, 179 (2016)
M. Fan, G.F.S. Andrade, A.G. Brolo, Anal. Chim. Acta. 693, 7 (2011)
L.B. Zhao, R. Huang, M.X. Bai, D.Y. Wu, Z.Q. Tian, J. Phys. Chem. C 115, 4174–4183 (2011). https://doi.org/10.1021/jp1117135
A. Mühlig, D. Cialla-May, J. Popp, J. Phys. Chem. C 121, 2323–2332 (2017). https://doi.org/10.1021/acs.jpcc.6b09368
N. Maiti, S. Thomas, J.A. Jacob, R. Chadha, T. Mukherjee, S. Kapoor, J. Colloid Interface Sci. 380, 141–149 (2012). https://doi.org/10.1016/j.jcis.2012.04.071
G. Díaz-Mirón, M.A. Sánchez, D.M. Chemes, R.M.S. Álvarez, J. Raman Spectrosc. 49(4), 638–650 (2017). https://doi.org/10.1002/jrs.5321
N. Leopold, B. Lendl, J. Phys. Chem. B 107(24), 5723–5727 (2003). https://doi.org/10.1021/jp027460u
L. Zhang, Appl. Surf. Sci. 270, 292–294 (2013). https://doi.org/10.1016/j.apsusc.2013.01.014
M. Lee, K. Oh, H.K. Choi, S.G. Lee, H.J. Youn, H.L. Lee, D.H. Jeong, ACS Sens. 3(1), 151–159 (2018). https://doi.org/10.1021/acssensors.7b00782
C. Novara, S.D. Marta, A. Virga, A. Lamberti, A. Angelini, A. Chiadò, P. Rivolo, F. Geobaldo, V. Sergo, A. Bonifacio, F. Giorgis, J. Phys. Chem. C 120(30), 16946–16953 (2016). https://doi.org/10.1021/acs.jpcc.6b03852
C. Zong, M. Xu, L.J. Xu, T. Wei, X. Ma, X.S. Zheng, R. Hu, B. Ren, Chem. Rev. 118, 4946–4980 (2018). https://doi.org/10.1021/acs.chemrev.7b00668
B. Hu, D.W. Sun, H. Pu, Q. Wei, Talanta 217, 120998 (2020). https://doi.org/10.1016/j.talanta.2020.120998
A. Savitzky, M.J.E. Golay, Anal. Chem. 36(8), 1627–1639 (1964). https://doi.org/10.1021/ac60214a047
M.L. Rizzato, A.L. Picone, R.M. Romano, Talanta Open. Volume. 7, 100223 (2023). https://doi.org/10.1016/j.talo.2023.100223
N. Hussain, H. Pu, D.W. Sun, Food Chem. 350, 129025, 1–11 (2021). https://doi.org/10.1016/j.foodchem.2021.129025
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Jr. Montgomery, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Revision E.01, Gaussian, Inc., Wallingford CT (2003)
A.N. Dominguez, G.E. Emmert, D.M. Gil, R.M.S. Álvarez, Spectrochim. Acta, Part A 259, 119888 (2021). https://doi.org/10.1016/j.saa.2021.119888
L. Zhao, L. Jensen, G.C. Schatz, J. Am. Chem. Soc. 128(9), 2911–2919 (2006). https://doi.org/10.1021/ja0556326
Y. Qi, Y. Hu, M. Xie, D. Xing, H. Gu, J. Raman Spectrosc. 42(6), 1287–1293 (2011). https://doi.org/10.1002/jrs.2864
A. Parameswari, R.M. Asath, R. Premkumar, A.M.F. Benial, J. Mol. Struct. 1138, 102–109 (2017). https://doi.org/10.1016/j.molstruc.2017.03.014
F.E. Gallegos, L.M. Meneses, S.A. Cuesta, J.C. Santos, J. Arias, P. Carrillo, F. Pilaquinga, ACS Omega. 7(6), 4750–4756 (2022). https://doi.org/10.1021/acsomega.1c04149
M.C. Daza, J.A. Dobado, J.M. Molina, P. Salvador, M. Duran, J.L. Villaveces, J. Chem. Phys. 110(24), 11806–11813 (1999). https://doi.org/10.1063/1.479166
S.S. Xantheas, J. Chem. Phys. 104(21), 8821–8824 (1996). https://doi.org/10.1063/1.471605
S. Singh, S.K. Srivastava, D.K. Singh, RSC Adv. 3(13), 4381–4390 (2013). https://doi.org/10.1039/C3RA22730H
R.M.S. Álvarez, C.O. Della Védova, H.G. Mack, R.N. Farías, P. Hildebrandt, Eur. Biophys. J. 31(6), 448–453 (2002). https://doi.org/10.1007/s00249-002-0238-y
R. Dennington, I.I.T. Keith, J. Millam, K. Eppinnett, W.L. Hovell, R. Gilliland, GaussView version 4.1, Semichem, Shawnee Mission, KS, USA. (2003)
J. Krajczewski, K. Kołataj, A. Kudelski, RSC Adv. 7(28), 17559–17576 (2017). https://doi.org/10.1039/C7RA01034F
S. Agnihotri, S. Mukherji, S. Mukherji, RSC Adv. 4(8), 3974–3983 (2014). https://doi.org/10.1039/C3RA44507K
D. Ramirez, F. Jaramillo, DYNA 83(198), 165–170 (2016) https://doi.org/10.15446/dyna.v83n198.48707
F. Zapata, F. Ortega-Ojeda, C. García-Ruiz, M. González-Herráez, Sensors. 18(7), 2196 (2018)
Z. Liang, Y. Chu, M. Gen, C.K. Chan, Atmos. Chem. Phys. 22, 3017–3044 (2022). https://doi.org/10.5194/acp-22-3017-2022
Servicio Nacional de Sanidad y Calidad Agroalimentaria, Tratamientos químicos obligatorios para el control de mancha negra de los cítricos (Phyllosticta citricarpa), https://www.argentina.gob.ar/sites/default/files/tratamientos_quimicos_obligatorios_para_el_control_de_mancha_negra_de_los_citricos.pdf. Accessed 7 April 2023
V. Kumar, K. Kaur, G.K. Gupta, A.K. Sharma, Eur. J. Med. Chem. 69, 735–753 (2013). https://doi.org/10.1016/j.ejmech.2013.08.053
Acknowledgements
This work was supported by Universidad Nacional de Tucumán, Argentina [Grant PIUNT2018 D604 to R.M.S.A.]. A.N.D. and L.E.J. are grateful to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for their Doctoral fellowships. R.M.S.A. is a career researcher of CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dominguez, A.N., Jimenez, L.E. & Álvarez, R.M.S. Rapid detection of pyraclostrobin fungicide residues in lemon with surface-enhanced Raman spectroscopy. Food Measure 17, 6350–6362 (2023). https://doi.org/10.1007/s11694-023-02131-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02131-z