Abstract
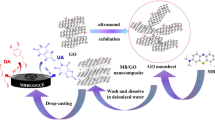
The current effort benefited from a simple hydrothermal production protocol to fabricate a nanocomposite using graphene oxide nanosheets anchored with manganese dioxide nanorods (MnO2 NRs-GO) that was applied to construct a sensitive electrochemical catechol sensing system. As-produced nanocomposite was characterized by Field emission-scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and Fourier transformed-infrared spectroscopy (FT-IR), which was then drop-casted on glassy carbon electrode (MnO2 NRs-GO/GCE) for sensor application. The MnO2 NRs-GO/GCE showed remarkable electrocatalytic capacity for the redox reaction of catechol, with minimized redox overpotentials and boosted voltammetric peak currents. As-fabricated sensor displayed an admirable electrocatalytic behavior to catechol, with a narrow limit of detection (0.02 µΜ), a broad linear range (0.5–400.0 µM) and an impressive sensitivity (0.0503 µA/µΜ). The MnO2 NRs-GO/GCE was also successful in sensing catechol in real specimens, with appreciable recovery rates.







Similar content being viewed by others
References
Y.G. Sun, H. Cui, Y.H. Li, Q.X. Lin, Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta 53, 661–666 (2000)
G. Wang, X. He, F. Zhou, Z. Li, B. Fang, X. Zhang, L. Wang, Application of gold nanoparticles/TiO2 modified electrode for the electrooxidative determination of catechol in tea samples. Food Chem. 135, 446–451 (2012)
A. Padmanaban, G. Murugadoss, N. Venkatesh, S. Hazra, M.R. Kumar, R. Tamilselvi, P. Sakthivel, Electrochemical determination of harmful catechol and rapid decolorization of textile dyes using ceria and tin doped ZnO nanoparticles. J. Environ. Chem. Eng. 9, 105976 (2021)
Y. Xu, Y. Yu, S. Xue, X. Ma, H. Tao, Innovative electrochemical sensor based on graphene oxide aerogel wrapped copper centered metal-organic framework to detect catechol. J. Electroanal. Chem. 899, 115686 (2021)
B.P. Sanjay, N.K. Swamy, S.R. Yashas, S. Sandeep, Design of an electrochemical sensor using 2D sheet-like Cu@g-C3N4 transducer matrix for electroanalysis of catechol. J. Electrochem. Soc. 168, 076511 (2021)
C. Liu, J. Hu, S. Biswas, F. Zhu, J. Zhan, G. Wang, Y. Wang, Surface-enhanced Raman scattering of phenols and catechols by a molecular analogue of titanium dioxide. Anal. Chem. 92, 5929–5936 (2020)
P. Nagaraja, R.A. Vasantha, K.R. Sunitha, A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 55, 1039–1046 (2001)
X. Li, J. Pan, F. Yang, J. Feng, J. Mo, Z. Chen, Simple amperometric detector for microchip capillary electrophoresis, and its application to the analysis of dopamine and catechol. Microchim. Acta 174, 123–130 (2011)
L. Zhao, B. Lv, H. Yuan, Z. Zhou, D. Xiao, A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 7, 578–588 (2007)
A. Wolf, R. Hüttl, G. Wolf, Enzymatic reactions for the calorimetric detection of phenolic compounds. J. Therm. Anal. Calorim. 61, 37–44 (2000)
E.L.B. Lourenço, A. Ferreira, E. Pinto, M. Yonamine, S.H.P. Farsky, On-fiber derivatization of SPME extracts of phenol, hydroquinone and catechol with GC-MS detection. Chromatographia 63, 175–179 (2006)
G. Marrubini, E. Calleri, T. Coccini, A.F. Castoldi, L. Manzo, Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column HPLC with fluorimetric detection. Chromatographia 62, 25–31 (2005)
L. Zhao, J. Yu, S. Yue, L. Zhang, Z. Wang, P. Guo, Q. Liu, Nickel oxide/carbon nanotube nanocomposites prepared by atomic layer deposition for electrochemical sensing of hydroquinone and catechol. J. Electroanal. Chem. 808, 245–251 (2018)
W. Si, W. Lei, Y. Zhang, M. Xia, F. Wang, Q. Hao, Electrodeposition of graphene oxide doped poly (3,4-ethylenedioxythiophene) film and its electrochemical sensing of catechol and hydroquinone. Electrochim. Acta 85, 295–301 (2012)
B. Farhadi, M. Ebrahimi, A. Morsali, Microextraction and Determination Trace Amount of Propranolol in Aqueous and Pharmaceutical Samples with Oxidized Multiwalled Carbon Nanotubes. Chem. Methodol. 5, 227–233 (2021)
M. Alizadeh, E. Demir, N. Aydogdu, N. Zare, F. Karimi, S.M. Kandomal, H. Rokni, Y. Ghasemi, Recent advantages in electrochemical monitoring for the analysis of amaranth and carminic acid food colors. Food Chem. Toxicol. 163, 112929 (2022)
S. Tajik, H.F. Beitollahi, M. Garkani-Nejad, P. Safaei, Mohammadzadeh-Jahani, Electrochemical sensing of Sudan I using the modified graphite screen-printed electrode. Int. J. Environ. Anal. Chem. 102, 1477–1490 (2022)
M. Ghalkhani, N. Zare, F. Karimi, C. Karaman, M. Alizadeh, Y. Vasseghian, Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food Chem. Toxicol. 161, 112830 (2022)
A. Hosseini Fakhrabad, R. Sanavi Khoshnood, M.R. Abedi, M. Ebrahimi, Fabrication a composite carbon paste electrodes (CPEs) modified with multi-wall carbon nano-tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 3, 627–634 (2021)
T. Eren, N. Atar, M.L. Yola, H. Karimi-Maleh, A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 185, 430–436 (2015)
N.P. Shetti, S.J. Malode, R.S. Malladi, S.L. Nargund, S.S. Shukla, T.M. Aminabhavi, Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode. Microchem J. 146, 387–392 (2019)
A. Shamsi, F. Ahour, Electrochemical sensing of thioridazine in human serum samples using modified glassy carbon electrode. Adv. J. Chem. A 4, 22–31 (2020)
F. Garkani-Nejad, H. Beitollahi, R. Alizadeh, Sensitive determination of hydroxylamine on ZnO nanorods/graphene oxide nanosheets modified graphite screen printed electrode. Anal. Bioanal Electrochem. 9, 134–144 (2017)
F. Karimi, E. Demir, N. Aydogdu, M. Shojaei, M.A. Taher, P.N. Asrami, M. Alizadeh, Y. Ghasemi, S. Cheraghi, Advancement in electrochemical strategies for quantification of Brown HT and Carmoisine (Acid Red 14) Drom Azo Dyestuff class. Food Chem. Toxicol. 165, 113075 (2022)
M.R. Aflatoonian, B. Aflatoonian, R. Alizadeh, R. Abbasi, Rayeni, Voltammetric determination of zolpidem by using glassy carbon electrode modified with Ag/ZnO nanoplates. Eurasian Chem. Commun. 2, 35–43 (2020)
L. Wang, Y. Wang, Q. Zhuang, Simple self-referenced ratiometric electrochemical sensor for dopamine detection using electrochemically pretreated glassy carbon electrode modified by acid-treated multiwalled carbon nanotube. J. Electroanal. Chem. 851, 113446 (2019)
S. Sharma, Glassy carbon: A promising material for micro-and nanomanufacturing. Materials 11, 1857 (2018)
H. Moradpour, H. Beitollahi, F. Garkani-Nejad, A. Di Bartolomeo, Glassy carbon electrode modified with n-doped reduced graphene oxide sheets as an effective electrochemical sensor for amaranth detection. Materials 15, 3011 (2022)
J.A. Buledi, N. Mahar, A. Mallah, A.R. Solangi, I.M. Palabiyik, N. Qambrani, F. Karimi, Y. Vasseghian, H. Karimi-Maleh, Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: a potential method for environmental remediation. Food Chem. Toxicol. 161, 112843 (2022)
L. de Souza Vieira, A review on the use of glassy carbon in advanced technological applications. Carbon 186, 282–302 (2022)
P. Prasad, N.Y. Sreedhar, Effective SWCNTs/Nafion electrochemical sensor for detection of dicapthon pesticide in water and agricultural food samples. Chem. Methodol. 2, 277–290 (2018)
W.E. Van der Linden, J.W. Dieker, Glassy carbon as electrode material in electro-analytical chemistry. Anal. Chim. Acta 119, 1–24 (1980)
L. Zheng, J.F. Song, Curcumin multi-wall carbon nanotubes modified glassy carbon electrode and its electrocatalytic activity towards oxidation of hydrazine. Sens. Actuators B: Chem. 135, 650–655 (2009)
H. Mahmoudi-Moghaddam, S. Tajik, H. Beitollahi, A new electrochemical DNA biosensor based on modified carbon paste electrode using graphene quantum dots and ionic liquid for determination of topotecan. Microchem J. 150, 104085 (2019)
S. Mehdizadeh, N. Ghasemi, M. Ramezani, K. Mahanpoor, Biosynthesis of silver nanoparticles using malva sylvestris flower extract and its antibacterial and catalytic activity. Chem. Methodol. 5, 356–366 (2021)
M.M. Foroughi, H. Beitollahi, S. Tajik, A. Akbari, R. Hosseinzadeh, Electrochemical determination of N-acetylcysteine and folic acid in pharmaceutical and biological samples using a modified carbon nanotube paste electrode. Int. J. Electrochem. 9, 8407 (2014)
A.A. Ensafi, E. Khoddami, B. Rezaei, H. Karimi-Maleh, p-Aminophenol–multiwall carbon nanotubes–TiO2 electrode as a sensor for simultaneous determination of penicillamine and uric acid. Colloids Surf. B: Biointerfaces 81, 42–49 (2010)
M. Pirozmand, A. Nezhadali, M. Payehghadr, L. Saghatforoush, Ultratrace determination of cadmium ion in petro-chemical sample by a new modified carbon paste electrode as voltammetric sensor. Eurasian Chem. Commun. 2, 1021–1032 (2020)
S. Cheraghi, M.A. Taher, H. Karimi-Maleh, F. Karimi, M. Shabani-Nooshabadi, M. Alizadeh, A. Al-Othman, N. Erk, P.K.Y. Raman, C. Karaman, Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 287, 132187 (2022)
S. Tajik, H. Beitollahi, S. Shahsavari, F. Garkani-Nejad, Simultaneous and selective electrochemical sensing of methotrexate and folic acid in biological fluids and pharmaceutical samples using Fe3O4/ppy/Pd nanocomposite modified screen printed graphite electrode. Chemosphere 291, 132736 (2022)
S. Li, J. Fan, S. Li, Y. Ma, J. Wu, H. Jin, Z. Guo, In situ-grown Co3O4 nanorods on carbon cloth for efficient electrocatalytic oxidation of urea. J. Nanostructure Chem. 11, 735–749 (2021)
H. Karimi-Maleh, M. Sheikhshoaie, I. Sheikhshoaie, M. Ranjbar, J. Alizadeh, N.W. Maxakato, A. Abbaspourrad, A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode. New. J. Chem. 43, 2362–2367 (2019)
P. Nasehi, M.S. Moghaddam, N. Rezaei-savadkouhi, M. Alizadeh, M.N. Yazdani, H. Agheli, Monitoring of Bisphenol A in water and soft drink products using electrochemical sensor amplified with TiO2-SWCNTs and ionic liquid. J. Food Meas. Charact. 16, 2440–2445 (2022)
H. Beitollahi, M.A. Khalilzadeh, S. Tajik, M. Safaei, K. Zhang, H.W. Jang, M. Shokouhimehr, Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 5, 2049–2059 (2020)
A. Khoobi, A.M. Attaran, M. Yousofi, M. Enhessari, A sensitive lead titanate nano-structured sensor for electrochemical determination of pentoxifylline drug in real samples. J. Nanostructure Chem. 9, 29–37 (2019)
C. Karaman, Orange peel derived-nitrogen and sulfur Co‐doped carbon dots: a nano‐booster for enhancing ORR electrocatalytic performance of 3D graphene networks. Electroanalysis 33, 1356–1369 (2021)
H. Jafarzadeh, C. Karaman, A. Güngör, O. Karaman, P.L. Show, P. Sami, A.A. Mehrizi, Hydrogen production via sodium borohydride hydrolysis catalyzed by cobalt ferrite anchored nitrogen-and sulfur co-doped graphene hybrid nanocatalyst: Artificial neural network modeling approach. Chem. Eng. Res. Des. 183, 557–566 (2022)
W.H. Elobeid, A.A. Elbashir, Development of chemically modified pencil graphite electrode based on benzo-18-crown-6 and multi-walled CNTs for determination of lead in water samples. Prog Chem. Biochem. Res. 2, 24–33 (2019)
H. Karimi-Maleh, C. Karaman, O. Karaman, F. Karimi, Y. Vasseghian, L. Fu, M. Baghayeri, J. Rouhi, P. Senthil Kumar, P.L. Show, S. Rajendran, A.L. Sanati, A. Mirabi. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass-derived activated carbon frameworks: a promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostructure Chem. 12, 429–439 (2022)
H. Karimi-Maleh, A.F. Shojaei, K. Tabatabaeian, F. Karimi, S. Shakeri, R. Moradi, Simultaneous determination of 6-mercaptopruine, 6-thioguanine and dasatinib as three important anticancer drugs using nanostructure voltammetric sensor employing Pt/MWCNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate. Biosens. Bioelectron. 86, 879–884 (2016)
H. Sadeghi, S.A. Shahidi, S. Naghizadeh Raeisi, A. Ghorbani-HasanSaraei, F. Karimi, Electrochemical determination of folic acid in fruit juices samples using electroanalytical sensor amplified with CuO/SWCNTs and 1-butyl-2,3-dimethylimidazolium hexafluorophosphate. Chem. Methodol. 4, 743–753 (2020)
M. Saha, S. Das, (2014) Electrochemical detection of L-serine and L-phenylalanine at bamboo charcoal–carbon nanosphere electrode. J. Nanostructure Chem. 4, 1–9 (2014)
A. Akca, O. Karaman, C. Karaman, Mechanistic insights into catalytic reduction of N2O by CO over Cu-embedded graphene: a density functional theory perspective. ECS J. Solid State Sci. 10, 041003 (2021)
N.H. Khand, I.M. Palabiyik, J.A. Buledi, S. Ameen, A.F. Memon, T. Ghumro, A.R. Solangi, Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J. Nanostructure Chem. 11, 455–468 (2021)
H. Karimi-Maleh, R. Darabi, M. Shabani-Nooshabadi, M. Baghayeri, F. Karimi, J. Rouhi, C. Karaman, Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 162, 112907 (2022)
J.B. Raoof, R. Ojani, H. Beitollahi, Electrocatalytic determination of ascorbic acid at chemically modified carbon paste electrode with 2, 7-bis (ferrocenyl ethynyl) fluoren-9-one. Int. J. Electrochem. Sci. 2, 534–548 (2007)
G. Vinodha, P.D. Shima, L. Cindrella, Mesoporous magnetite nanoparticle-decorated graphene oxide nanosheets for efficient electrochemical detection of hydrazine. J. Mater. Sci. 54, 4073–4088 (2019)
S. Immanuel, R. Sivasubramanian, Electrochemical studies of NADH oxidation on chemically reduced graphene oxide nanosheets modified glassy carbon electrode. Mater. Chem. Phys. 249, 123015 (2020)
S. Ahmad, M.H. Ayoub, A.M. Khan, A. Waseem, M. Yasir, M.S. Khan, A.J. Shaikh, Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf. A: Physicochem Eng. Asp 647, 129057 (2022)
S. Nagarani, G. Sasikala, K. Satheesh, M. Yuvaraj, R. Jayavel, Synthesis and characterization of binary transition metal oxide/reduced graphene oxide nanocomposites and its enhanced electrochemical properties for supercapacitor applications. J. Mater. Sci. Mater. Electron. 29, 11738–11748 (2018)
H. Beitollahi, S. Tajik, A. Di Bartolomeo, Application of MnO2 nanorod–ionic liquid modified carbon paste electrode for the voltammetric determination of sulfanilamide. Micromachines 13, 598 (2022)
M. Lübke, A. Sumboja, L. McCafferty, C.F. Armer, A.D. Handoko, Y. Du, J.A. Darr, Transition-metal‐doped α‐MnO2 nanorods as bifunctional catalysts for efficient oxygen reduction and evolution reactions. Chem. Select 3, 2613–2622 (2018)
K. Ahmad, A. Mohammad, S.M. Mobin, Hydrothermally grown α-MnO2 nanorods as highly efficient low cost counter-electrode material for dye-sensitized solar cells and electrochemical sensing applications. Electrochim. Acta 252, 549–557 (2017)
J.J. Feng, P.P. Zhang, A.J. Wang, Y. Zhang, W.J. Dong, J.R. Chen, One-pot hydrothermal synthesis of uniform β-MnO2 nanorods for nitrite sensing. J. Colloid Interface Sci. 359, 1–8 (2011)
F. Garkani-Nejad, M.H. Asadi, I. Sheikhshoaie, Z. Dourandish, R. Zaeimbashi, H. Beitollahi, Construction of modified screen-printed graphite electrode for the application in electrochemical detection of sunset yellow in food samples. Food Chem. Toxicol. 166, 113243 (2022)
V. Ganesan, C. Louis, S.P. Damodaran, Graphene oxide wrapped magnetite nanoclusters: a recyclable functional hybrid for fast and highly efficient removal of organic dyes from wastewater. J. Environ. Chem. Eng. 6, 2176–2190 (2018)
Z.S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, H.M. Cheng, Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 4, 3187–3194 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karami-Kolmoti, P., Beitollahi, H. & Modiri, S. Voltammetric detection of catechol in real samples using MnO2 nanorods-graphene oxide nanocomposite modified electrode. Food Measure 17, 1974–1984 (2023). https://doi.org/10.1007/s11694-022-01692-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01692-9