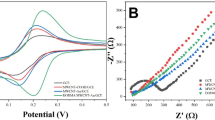
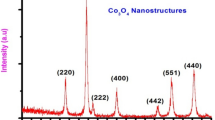
Abstract
Quercetin (Qu) is a most active biological flavonoid and it has a very wide spectrum of potential applications. Herein, we have synthesized ionic liquid assisted Co3O4 nanostructures through an aqueous chemical growth method and fabricated a Co3O4 modified GCE as an electrochemical sensor for the sensitive detection and determination of Qu. The proposed electrochemical sensor was not only prepared with a very easy, simple and cheap method but it was also found to be very selective, sensitive and highly stable for the detection of Qu in standard solutions as well as in real food samples like onion, honey and green tea. The prepared electrochemical sensor has shown an excellent electrochemical response for Qu with a wide range of detection from 0.01 to 3 µM. The oxidation current response of Qu on Co3O4 modified GCE was found 4 times higher than the response of bare GCE which is due to the high conductivity, tremendous catalytic ability and large surface area of Co3O4 nanostructures. The limit of detection (LOD) and the limit of quantification (LOQ) for Co3O4/GCE sensor was calculated and found to be 0.0002 µM and 0.0007 µM respectively. While, the amount of Qu in real samples was found to be 5.367 µg/mL in honey, 15.58 µg/g in onion and 3.473 mg/g in green tea respectively. In comparison to the previously reported sensors, the prepared Co3O4/GCE sensor has shown a higher electrocatalytic capability, remarkable stability, super sensitivity and adequate selectivity for the determination of Qu in standard solutions as well as in real samples.










Similar content being viewed by others
References
S. Takahashi, H. Muguruma, N. Osakabe, H. Inoue, T. Ohsawa, Electrochemical determination with a long-length carbon nanotube electrode of quercetin glucosides in onion, apple peel, and tartary buckwheat. Food Chem. 300, 125189 (2019)
A. Saljooqi, T. Shamspur, A. Mostafavi, Fe3O4@ SiO2-PANI-Au nanocomposite prepared for electrochemical determination of quercetin in food samples and biological fluids. Electroanalysis (2019). https://doi.org/10.1002/elan.201900386
M. Kawser Hossain, A. Abdal Dayem, J. Han, Y. Yin, K. Kim, S. Kumar Saha, G.-M. Yang, H.Y. Choi, S.-G. Cho, Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 17(4), 569 (2016)
M. Skerget, P. Kotnik:, A.R. Hras, M. Simonic, M. Ha dolin. Z. Knez. Food Chem 89, 191 (2005)
A. Mondal, D. Rajalingam, T.K. Maity, Anti-inflammatory effect of O-methylated flavonol 2-(3, 4-dihydroxy-phenyl)-3, 5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J. Ethnopharmacol. 147(2), 525–529 (2013)
M. Saito, H. Hosoyama, T. Ariga, S. Kataoka, N. Yamaji, Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 46(4), 1460–1464 (1998)
D. Saritha, A. Koirala, M. Venu, G.D. Reddy, A.V.B. Reddy, B. Sitaram, G. Madhavi, K. Aruna, A simple, highly sensitive and stable electrochemical sensor for the detection of quercetin in solution, onion and honey buckwheat using zinc oxide supported on carbon nanosheet (ZnO/CNS/MCPE) modified carbon paste electrode. Electrochim. Acta 313, 523–531 (2019)
F.C. Bekkering, A.U. Neumann, J.T. Brouwer, R.S. Levi-Drummer, S.W. Schalm, Changes in anti-viral effectiveness of interferon after dose reduction in chronic hepatitis C patients: a case control study. BMC Gastroenterol. 1(1), 14 (2001)
R. Khani, R. Sheykhi, G. Bagherzade, An environmentally friendly method based on micro-cloud point extraction for determination of trace amount of quercetin in food and fruit juice samples. Food Chem. 293, 220–225 (2019)
Z. Zhou, C. Gu, C. Chen, P. Zhao, Y. Xie, J. Fei, An ultrasensitive electrochemical sensor for quercetin based on 1-pyrenebutyrate functionalized reduced oxide graphene/mercapto-β-cyclodextrin/Au nanoparticles composite film. Sens. Actuators B 288, 88–95 (2019)
P. Zhao, M. Ni, Y. Xu, C. Wang, C. Chen, X. Zhang, C. Li, Y. Xie, J. Fei, A novel ultrasensitive electrochemical quercetin sensor based on MoS2-carbon nanotube@ graphene oxide nanoribbons/HS-cyclodextrin/graphene quantum dots composite film. Sens. Actuators B 299, 126997 (2019)
S. Kumar, V. Lather, D. Pandita, Stability indicating simplified HPLC method for simultaneous analysis of resveratrol and quercetin in nanoparticles and human plasma. Food Chem. 197, 959–964 (2016)
R. Ravichandran, M. Rajendran, D. Devapiriam, Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 146, 472–478 (2014)
Y. Sun, T. Guo, Y. Sui, F. Li, Quantitative determination of rutin, quercetin, and adenosine in Flos Carthami by capillary electrophoresis. J. Sep. Sci. 26(12-13), 1203–1206 (2003)
C. Wang, Y. Zuo, Ultrasound-assisted hydrolysis and gas chromatography–mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 128(2), 562–568 (2011)
J.B. Raoof, R. Ojani, M. Amiri-Aref, M. Baghayeri, Electrodeposition of quercetin at a multi-walled carbon nanotubes modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of levodopa, uric acid and tyramine. Sens. Actuators B 166, 508–518 (2012)
M. Ghanei-Motlagh, M. Baghayeri, Determination of trace Tl (I) by differential pulse anodic stripping voltammetry using a novel modified carbon paste electrode. J. Electrochem. Soc. 167(6), 066508 (2020)
M. Nodehi, M. Baghayeri, R. Ansari, H. Veisi, Electrochemical quantification of 17α-ethinylestradiol in biological samples using a Au/Fe3O4@ TA/MWNT/GCE sensor. Mater. Chem. Phys. 244, 122687 (2020)
M. Nodehi, M. Baghayeri, R. Behazin, H. Veisi, Electrochemical aptasensor of bisphenol A constructed based on 3D mesoporous structural SBA-15-Met with a thin layer of gold nanoparticles. Microchem. J. 162, 105825 (2021)
M. Ghanei-Motlagh, M.A. Taher, M. Fayazi, M. Baghayeri, A. Hosseinifar, Non-enzymatic amperometric sensing of hydrogen peroxide based on vanadium pentoxide nanostructures. Journal of The Electrochemical Society 166(6), B367 (2019)
M. Baghayeri, R. Ansari, M. Nodehi, I. Razavipanah, H. Veisi, Label-free electrochemical bisphenol A aptasensor based on designing and fabrication of a magnetic gold nanocomposite. Electroanalysis 30(9), 2160–2166 (2018)
M. Baghayeri, R. Ansari, M. Nodehi, I. Razavipanah, H. Veisi, Voltammetric aptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@ gold nanocomposite. Microchim. Acta 185(7), 1–9 (2018)
M. Baghayeri, H. Beitollahi, A. Akbari, S. Farhadi, Highly sensitive nanostructured electrochemical sensor based on carbon nanotubes-Pt nanoparticles paste electrode for simultaneous determination of levodopa and tyramine. Russ. J. Electrochem. 54(3), 292–301 (2018)
M. Baghayeri, A. Sedrpoushan, A. Mohammadi, M. Heidari, A non-enzymatic glucose sensor based on NiO nanoparticles/functionalized SBA 15/MWCNT-modified carbon paste electrode. Ionics 23(6), 1553–1562 (2017)
N.H. Khand, I.M. Palabiyik, J.A. Buledi, S. Ameen, A.F. Memon, T. Ghumro, A.R. Solangi, Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J. Nanostruct. Chem. (2021). https://doi.org/10.1007/s40097-020-00380-8
S. Kiranmai, Y.V.M. Reddy, M. Venu, C. Madhuri, K. Anitha, G. Madhavi, A.V. Reddy, Determination of Terazosin by using poly (Congo red) modified carbon paste electrode. Anal. Bioanal. Electrochem. 9(2), 154–163 (2017)
H. Karimi-Maleh, M. Alizadeh, Y. Orooji, F. Karimi, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V.K. Gupta, Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: a docking/experimental investigation. Ind. Eng. Chem. Res. 60(2), 816–823 (2021)
M. Khadem, F. Faridbod, P. Norouzi, A. Rahimi Foroushani, M.R. Ganjali, S.J. Shahtaheri, R. Yarahmadi, Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis 29(3), 708–715 (2017)
H. Karimi-Maleh, F. Karimi, Y. Orooji, G. Mansouri, A. Razmjou, A. Aygun, F. Sen, A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 10(1), 11699 (2020)
H. Karimi-Maleh, K. Cellat, K. Arıkan, A. Savk, F. Karimi, F. Şen, Palladium–nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 250, 123042 (2020)
J.A. Buledi, A.M. Zia-ul-Hassan Shah, A.R. Solangi, Current perspective and developments in electrochemical sensors modified with nanomaterials for environmental and pharmaceutical analysis. Curr. Anal. Chem. 17, 1–4 (2021)
F.A. Harraz, M. Faisal, A. Al-Salami, A.M. El-Toni, A. Almadiy, S. Al-Sayari, M. Al-Assiri, Silver nanoparticles decorated stain-etched mesoporous silicon for sensitive, selective detection of ascorbic acid. Mater. Lett. 234, 96–100 (2019)
K. Saksena, A. Shrivastava, R. Kant, Chiral analysis of ascorbic acid in bovine serum using ultrathin molecular imprinted polyaniline/graphite electrode. J. Electroanal. Chem. 795, 103–109 (2017)
X. Wu, Y. Xing, D. Pierce, J.X. Zhao, One-pot synthesis of reduced graphene oxide/metal (oxide) composites. ACS Appl. Mater. Interfaces 9(43), 37962–37971 (2017)
Y. Hei, X. Li, X. Zhou, J. Liu, M. Hassan, S. Zhang, Y. Yang, X. Bo, H.-L. Wang, M. Zhou, Cost-effective synthesis of three-dimensional nitrogen-doped nanostructured carbons with hierarchical architectures from the biomass of sea-tangle for the amperometric determination of ascorbic acid. Anal. Chim. Acta 1029, 15–23 (2018)
M.R. Ganjali, H. Salimi, S. Tajik, H. Beitollahi, M. Rezapour, B. Larijani, Application of Fe3O4@ SiO2/MWCNT film on glassy carbon electrode for the sensitive electroanalysis of levodopa. Int. J. Electrochem. Sci. 12, 5243–5253 (2017)
M.R. Ganjali, H. Beitollahi, R. Zaimbashi, S. Tajik, M. Rezapour, B. Larijani, Voltammetric determination of dopamine using glassy carbon electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 13(3), 2519–2529 (2018)
H. Baksh, J.A. Buledi, N.H. Khand, A.R. Solangi, A. Mallah, S.T. Sherazi, M.I. Abro, Ultra-selective determination of carbofuran by electrochemical sensor based on nickel oxide nanoparticles stabilized by ionic liquid. Monatsh. Chem. 151(11), 1689–1696 (2020)
J.A. Buledi, S. Ameen, N.H. Khand, A.R. Solangi, I.H. Taqvi, M.H. Agheem, Z. Wajdan, CuO nanostructures based electrochemical sensor for simultaneous determination of hydroquinone and ascorbic acid. Electroanalysis 32(7), 1600–1607 (2020)
S. Tajyani, A. Babaei, A new sensing platform based on magnetic Fe3O4@ NiO core/shell nanoparticles modified carbon paste electrode for simultaneous voltammetric determination of Quercetin and Tryptophan. J. Electroanal. Chem. 808, 50–58 (2018)
A.E. Vilian, P. Puthiaraj, C.H. Kwak, S.R. Choe, Y.S. Huh, W.-S. Ahn, Y.-K. Han, Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta 216, 181–187 (2016)
M.L. Yola, V.K. Gupta, T. Eren, A.E. Şen, N. Atar, A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim. Acta 120, 204–211 (2014)
S. Vladimirova, V. Krivetskiy, M. Rumyantseva, A. Gaskov, N. Mordvinova, O. Lebedev, M. Martyshov, P. Forsh, Co3O4 as p-type material for CO sensing in humid air. Sensors 17(10), 2216 (2017)
A. Numan, M.M. Shahid, F.S. Omar, K. Ramesh, S. Ramesh, Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection. Sens. Actuators B 238, 1043–1051 (2017)
S.A. Memon, D. Hassan, J.A. Buledi, A.R. Solangi, S.Q. Memon, I.M. Palabiyik, Plant material protected cobalt oxide nanoparticles: sensitive electro-catalyst for tramadol detection. Microchem. J. 159, 105480 (2020)
G. Wang, F. Zhu, J. Xia, L. Wang, Y. Meng, Y. Zhang, Preparation of Co3O4/carbon derived from ionic liquid and its application in lithium-ion batteries. Electrochim. Acta 257, 138–145 (2017)
Z. Song, Y. Zhang, W. Liu, S. Zhang, G. Liu, H. Chen, J. Qiu, Hydrothermal synthesis and electrochemical performance of Co3O4/reduced graphene oxide nanosheet composites for supercapacitors. Electrochim. Acta 112, 120–126 (2013)
R.K. Das, A.K. Golder, Co3O4 spinel nanoparticles decorated graphite electrode: Bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim. Acta 251, 415–426 (2017)
M. Antonietti, D. Kuang, B. Smarsly, Y. Zhou, Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 43(38), 4988–4992 (2004)
R. Sheldon, Catalytic reactions in ionic liquids. Chem. Commun. 23, 2399–2407 (2001)
J. Fuller, R.T. Carlin, R.A. Osteryoung, The room temperature ionic liquid 1-ethyl‐3‐methylimidazolium tetrafluoroborate: electrochemical couples and physical properties. J. Electrochem. Soc. 144(11), 3881 (1997)
W. Zheng, X. Liu, Z. Yan, L. Zhu, Ionic liquid-assisted synthesis of large-scale TiO2 nanoparticles with controllable phase by hydrolysis of TiCl4. ACS Nano 3(1), 115–122 (2009)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley, New York, 2008).
J. Shen, B. Yan, M. Shi, H. Ma, N. Li, M. Ye, One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 21(10), 3415–3421 (2011)
V. Vinothkumar, A. Sangili, S.M. Chen, P. Veerakumar, K.-C. Lin, Sr-doped NiO3 nanorods synthesized by simple sonochemical method as excellent materials for voltammetric determination of quercetin. New J. Chem. 44, 2821–2832 (2020)
W. Zhang, L. Zong, G. Geng, Y. Li, Y. Zhang, Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens. Actuators B 257, 1099–1109 (2018)
B. Xu, L. Yang, F. Zhao, B. Zeng, A novel electrochemical quercetin sensor based on Pd/MoS2-ionic liquid functionalized ordered mesoporous carbon. Electrochim. Acta 247, 657–665 (2017)
Funding
The authors are highly thankful to the Higher Education Commission of Pakistan for providing funds under the project “6714/Sindh/NRPU/R&D/HEC/HEC/2015”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khand, N.H., Solangi, A.R., Ameen, S. et al. A new electrochemical method for the detection of quercetin in onion, honey and green tea using Co3O4 modified GCE. Food Measure 15, 3720–3730 (2021). https://doi.org/10.1007/s11694-021-00956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00956-0