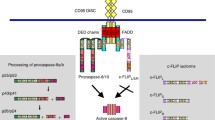
Abstract
Apoptotic death pathways are frequently activated by death ligand induction and subsequent activation of the membrane proximal signaling module. Death receptors cluster upon binding to death ligands, leading to formation of a membrane proximal death-inducing-signaling-complex (DISC). In this membrane proximal signalosome, initiator caspases (caspase 8) are processed resulting in activation of both type 1 and type 2 pathways of apoptosis signaling. How the type 1/type 2 choice is made is an important question in the systems biology of apoptosis signaling. In this study, we utilize a Monte Carlo based in silico approach to elucidate the role of membrane proximal signaling module in the type 1/type 2 choice of apoptosis signaling. Our results provide crucial mechanistic insights into the formation of DISC signalosome and caspase 8 activation. Increased concentration of death ligands was shown to correlate with increased type 1 activation. We also study the caspase 6 mediated system level feedback activation of apoptosis signaling and its role in the type 1/type 2 choice. Our results clarify the basis of cell-to-cell stochastic variability in apoptosis activation and ramifications of this issue is further discussed in the context of therapies for cancer and neurodegenerative disorders.







Similar content being viewed by others
References
Accordi B, Pillozzi S, Dell’Orto MC, Cazzaniga G, Arcangeli A, Kronnie GT, Basso G (2007) Hepatocyte growth factor receptor c-MET is associated with FAS and when activated enhances drug-induced apoptosis in pediatric B acute lymphoblastic leukemia with TEL-AML1 translocation. J Biol Chem 282(40):29384–29393. doi:10.1074/jbc.M706314200
Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK (2008) Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol 6(12):2831–2852. doi:10.1371/journal.pbio.0060299
Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout GB, Bahar I (2006) Bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys J 90(5):1546–1559. doi:10.1529/biophysj.105.068122
Brittain T, Skommer J, Raychaudhuri S, Birch N (2010) An antiapoptotic neuroprotective role for neuroglobin. Int J Mol Sci 11(6):2306–2321. doi:10.3390/ijms11062306
Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9(5):351–365. doi:10.1016/j.ccr.2006.03.027
Daniels RA, Turley H, Kimberley FC, Liu XS, Mongkolsapaya J, Ch’En P, Xu XN, Jin BQ, Pezzella F, Screaton GR (2005) Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res 15(6):430–438. doi:10.1038/sj.cr.7290311
Di Carlo M (2010) Beta amyloid peptide: from different aggregation forms to the activation of different biochemical pathways. Eur Biophys J 39(6):877–888. doi:10.1007/s00249-009-0439-8
Dussmann H, Rehm M, Concannon CG, Anguissola S, Wurstle M, Kacmar S, Voller P, Huber HJ, Prehn JH (2010) Single-cell quantification of Bax activation and mathematical modelling suggest pore formation on minimal mitochondrial Bax accumulation. Cell Death Differ 17(2):278–290. doi:10.1038/cdd.2009.123
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. doi:10.1080/01926230701320337
Falschlehner C, Emmerich CH, Gerlach B, Walczak H (2007) TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39(7–8):1462–1475. doi:10.1016/j.biocel.2007.02.007
Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN (2010) Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol 190(3):377–389. doi:10.1083/jcb.201002060
Gajate C, Mollinedo F (2011) Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Pat Anticancer Drug Discov 6(3):274–283. doi:10.2174/157489211796957766
George KS, Wu S (2012) Lipid raft: a floating island of death or survival. Toxicol Appl Pharmacol 259(3):311–319. doi:10.1016/j.taap.2012.01.007
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2(3):156–162. doi:10.1038/35004029
Gu C, Zhang J, Chen Y, Lei J (2011) A trigger model of apoptosis induced by tumor necrosis factor signaling. BMC Syst Biol 5(Suppl 1):S13. doi:10.1186/1752-0509-5-S1-S13
Gulbins E, Kolesnick R (2003) Raft ceramide in molecular medicine. Oncogene 22(45):7070–7077. doi:10.1038/sj.onc.1207146
Ho IA, Ng WH, Lam PY (2010) FasL and FADD delivery by a glioma-specific and cell cycle-dependent HSV-1 amplicon virus enhanced apoptosis in primary human brain tumors. Mol cancer 9:270. doi:10.1186/1476-4598-9-270
Hua F, Cornejo MG, Cardone MH, Stokes CL, Lauffenburger DA (2005) Effects of Bcl-2 levels on Fas signaling-induced caspase-3 activation: molecular genetic tests of computational model predictions. J Immunol 175(2):985–995
Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L). Proc Natl Acad Sci USA 96(26):14871–14876
Huang Y, Rich RL, Myszka DG, Wu H (2003) Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem 278(49):49517–49522. doi:10.1074/jbc.M310061200
Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, Strasser A, Kaufmann T (2009) XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460(7258):1035–1039. doi:10.1038/nature08229
Kim K, Fisher MJ, Xu SQ, el-Deiry WS (2000) Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res 6(2):335–346
Kurita S, Mott JL, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Roberts LR, Fernandez-Zapico ME, Gores GJ (2011) Hedgehog inhibition promotes a switch from type II to type I cell death receptor signaling in cancer cells. PLoS One 6(3):e18330. doi:10.1371/journal.pone.0018330
Leblanc AC (2013) Caspase-6 as a novel early target in the treatment of Alzheimer’s disease. Eur J Neurosci 37(12):2005–2018. doi:10.1111/ejn.12250
Lee JK, Lu S, Madhukar A (2010) Real-time dynamics of Ca2+, caspase-3/7, and morphological changes in retinal ganglion cell apoptosis under elevated pressure. PLoS One 5(10):e13437. doi:10.1371/journal.pone.0013437
Legembre P, Daburon S, Moreau P, Ichas F, de Giorgi F, Moreau JF, Taupin JL (2005) Amplification of Fas-mediated apoptosis in type II cells via microdomain recruitment. Mol Cell Biol 25(15):6811–6820. doi:10.1128/MCB.25.15.6811-6820.2005
Lopez-Araiza H, Ventura JL, Lopez-Diazguerrero NE, Gonzalez-Marquez H, Gutierrez-Ruiz MC, Zentella A, Konigsberg M (2006) Organ- and tissue-specific alterations in the anti-apoptotic protein Bcl-2 in CD1 female mice of different ages. Biogerontology 7(1):63–67. doi:10.1007/s10522-005-6038-x
Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P (2001) Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol 22(6):328–336
Meng XW, Peterson KL, Dai H, Schneider P, Lee SH, Zhang JS, Koenig A, Bronk S, Billadeau DD, Gores GJ, Kaufmann SH (2011) High cell surface death receptor expression determines type I versus type II signaling. J Biol Chem 286(41):35823–35833. doi:10.1074/jbc.M111.240432
Miyazaki T, Reed JC (2001) A GTP-binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nat Immunol 2(6):493–500. doi:10.1038/88684
Neumann L, Pforr C, Beaudouin J, Pappa A, Fricker N, Krammer PH, Lavrik IN, Eils R (2010) Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol Syst Biol 6:352. doi:10.1038/msb.2010.6
Newman MEJ, Barkema GT (1999) Monte Carlo methods in statistical physics. Oxford University Press, USA
Okazaki N, Asano R, Kinoshita T, Chuman H (2008) Simple computational models of type I/type II cells in Fas signaling-induced apoptosis. J Theor Biol 250(4):621–633. doi:10.1016/j.jtbi.2007.10.030
Ozoren N, El-Deiry WS (2002) Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia 4(6):551–557. doi:10.1038/sj.neo.7900270
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10(1):26–35. doi:10.1038/sj.cdd.4401186
Picarda G, Trichet V, Teletchea S, Heymann D, Redini F (2012) TRAIL receptor signaling and therapeutic option in bone tumors: the trap of the bone microenvironment. Am J Cancer Res 2(1):45–64
Picone P, Carrotta R, Montana G, Nobile MR, San Biagio PL, Di Carlo M (2009) Abeta oligomers and fibrillar aggregates induce different apoptotic pathways in LAN5 neuroblastoma cell cultures. Biophys J 96(10):4200–4211. doi:10.1016/j.bpj.2008.11.056
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ (2008) Efficient tumour formation by single human melanoma cells. Nature 456(7222):593–598. doi:10.1038/nature07567
Raychaudhuri S (2010) A minimal model of signaling network elucidates cell-to-cell stochastic variability in apoptosis. PLoS One 5(8):e11930. doi:10.1371/journal.pone.0011930
Raychaudhuri S (2013) Kinetic Monte Carlo simulation in biophysics and systems biology. In: Chan WKV (ed) Theory and applications of Monte Carlo simulations. InTech, Rijeka, Croatia
Raychaudhuri S, Das SC (2013) Low probability activation of Bax/Bak can induce selective killing of cancer cells by generating heterogeneoity in apoptosis. J Healthc Eng 4:47–66
Raychaudhuri S, Raychaudhuri SC (2013) Monte Carlo study elucidates the type 1/type 2 choice in apoptotic death signaling in healthy and cancer cells. Cells 2(2):361–392. doi:10.3390/cells2020361
Raychaudhuri S, Willgohs E, Nguyen TN, Khan EM, Goldkorn T (2008) Monte Carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys J 95(8):3559–3562. doi:10.1529/biophysj.108.135483
Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T (2010) Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis 15(4):401–411. doi:10.1007/s10495-009-0436-5
Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS (2001) Structural basis for the inhibition of caspase-3 by XIAP. Cell 104(5):791–800
Safa AR, Pollok KE (2011) Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers 3(2):1639–1671. doi:10.3390/cancers3021639
Sanlioglu AD, Dirice E, Aydin C, Erin N, Koksoy S, Sanlioglu S (2005) Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC cancer 5:54. doi:10.1186/1471-2407-5-54
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17(6):1675–1687. doi:10.1093/emboj/17.6.1675
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274(32):22532–22538
Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, Robinson H, Salvesen GS, Schwarzenbacher R, Riedl SJ (2009) The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 457(7232):1019–1022. doi:10.1038/nature07606
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277(5327):818–821
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y (2003) Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell 11(2):519–527
Shirley S, Morizot A, Micheau O (2011) Regulating TRAIL receptor-induced cell death at the membrane: a deadly discussion. Recent Pat Anticancer Drug Discov 6(3):311–323. doi:10.2174/157489211796957757
Skommer J, Brittain T, Raychaudhuri S (2010) Bcl-2 inhibits apoptosis by increasing the time-to-death and intrinsic cell-to-cell variations in the mitochondrial pathway of cell death. Apoptosis 15(10):1223–1233. doi:10.1007/s10495-010-0515-7
Skommer J, Das SC, Nair A, Brittain T, Raychaudhuri S (2011a) Nonlinear regulation of commitment to apoptosis by simultaneous inhibition of Bcl-2 and XIAP in leukemia and lymphoma cells. Apoptosis 16(6):619–626. doi:10.1007/s10495-011-0593-1
Skommer J, Raychaudhuri S, Wlodkowic D (2011b) Timing is everything: stochastic origins of cell-to-cell variability in cancer cell death. Front Biosci 16:307–314
Song JH, Tse MC, Bellail A, Phuphanich S, Khuri F, Kneteman NM, Hao C (2007) Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer Res 67(14):6946–6955. doi:10.1158/0008-5472.CAN-06-3896
Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK (2009) Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459(7245):428–432. doi:10.1038/nature08012
Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM (2002) Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem 277(13):11345–11351. doi:10.1074/jbc.M109893200
Sun Z, Ma X, Yang H, Zhao J, Zhang J (2012) Brain-derived neurotrophic factor prevents beta-amyloid-induced apoptosis of pheochromocytoma cells by regulating Bax/Bcl-2 expression. Neural Regen Res 7(5):347–351
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K (2001) Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 7(1):94–100. doi:10.1038/83416
Thome CH, dos Santos GA, Ferreira GA, Scheucher PS, Izumi C, Leopoldino AM, Simao AM, Ciancaglini P, de Oliveira KT, Chin A, Hanash SM, Falcao RP, Rego EM, Greene LJ, Faca VM (2012) Linker for activation of T-cell family member2 (LAT2) a lipid raft adaptor protein for AKT signaling, is an early mediator of alkylphospholipid anti-leukemic activity. Mol Cell Proteomics 11(12):1898–1912. doi:10.1074/mcp.M112.019661
Xiao W, Ishdorj G, Sun J, Johnston JB, Gibson SB (2011) Death receptor 4 is preferentially recruited to lipid rafts in chronic lymphocytic leukemia cells contributing to tumor necrosis related apoptosis inducing ligand-induced synergistic apoptotic responses. Leuk Lymphoma 52(7):1290–1301. doi:10.3109/10428194.2011.567317
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raychaudhuri, S., Raychaudhuri, S.C. Death ligand concentration and the membrane proximal signaling module regulate the type 1/type 2 choice in apoptotic death signaling. Syst Synth Biol 8, 83–97 (2014). https://doi.org/10.1007/s11693-013-9124-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11693-013-9124-4