Abstract
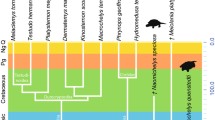
The cervical system of extant penguins (Aves: Sphenisciformes) is organised into morphological modules, each with its biomechanical function. Indeed, for these marine pelagic birds to acquire hydrodynamic morphology, the folding of the neck is essential. Despite a common general structure, the cervical vertebrae exhibit morphological differences depending on their positioning. These characteristics are identified as apparent cases of complete natural homeotic transformations—therefore, the composition of some modules varies. Two types of complete cervical homeoses are identified between species, but the second type can also occur within some species when the post hatching development is considered. The fossil material analysed here makes it apparent that the two modular configurations characterising the anterior part of the neck—a consequence of the first homeosis—existed 36 My and 25 My ago, for one, and circa 10 My ago, for the other. These comparisons also reveal a clear differentiation in vertebral features between the fossil species of the Oligocene–Miocene ages and the more recent and extant penguins. Ultimately, these observations make the proposal of a hypothesis in relation to the ontogenetic influence of Hox genes, and their regulators, based on the changes observed in the cervical segment of Sphenisciformes.










Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Notes
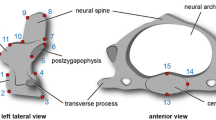
Adult A. patagonicus possess tiny ventral apophyses on their transitional vertebra but these cannot be regarded as the strong processus caroticus characteristic of module 5 in all species. This is why the word “true” is used.
References
Abu-Abed, S., Dolle, P., Metzger, D., Beckett, B., Chambon, P., & Petkovich, M. (2000). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes and Development, 15, 226–240.
Acosta Hospitaleche, C., Tambussi, C., Donato, M., & Cozzuol, M. (2007). A new Miocene penguin from Patagonia and its phylogenetic relationships. Acta Palaeontologica Polonica, 52(2), 299–314.
Alexander, T., Nolte, C., & Krumlauf, R. (2009). Hox Genes and segmentation of the hindbrain and axial skeleton. Annual Review of Cell and Developmental Biology, 25, 431–456.
Aubin, J., & Jeanotte, L. (2001). Implication des gènes Hox dans les processus d’organogenèse chez les mammifères. Medicine and Science, 17, 54–62.
Baumel, J. J., King, A. S., Breazile, J. E., Evans, H. E., & Vanden Berge, J. C. (1993). Handbook of avian anatomy: Nomina anatomica avium (2nd ed.). Cambridge, MA: Publications of the Nuttall Ornithological Club.
Bertelli, S., Giannini, N. P., & Ksepka, D. T. (2006). Redescription and phylogenetic position of the early Miocene penguin Paraptenodytes antarcticus from Patagonia. American Museum Novitates, 3525, 1–36.
Burke, A. C., Nelson, C. E., Morgan, B. A., & Tabin, C. (1995). Hox genes and the evolution of vertebrate axial morphology. Development, 121, 333–346.
Carapuço, M., Nóvoa, A., Bobola, N., & Mallo, M. (2005). Hox genes specify vertebral types in the presomitic mesoderm. Genes and Development, 19, 2116–2121.
Clarke, J. A., Ksepka, D. T., Stucchi, M., Urbina, M., Giannini, N., Bertelli, D., et al. (2007). Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proceedings of the National Academy of Science, 104(28), 11545–11550.
Colon, R. A. (1995). Retinoic acid and pattern formation in vertebrates. Trends in Genetics, 11(8), 314–319.
Cordes, R., Schuster-Gossler, K., Serth, K., & Gossler, A. (2004). Specification of vertebral identity is coupled to Notch signaling and the segmentation clock. Development, 131, 1221–1233.
Deschamps, J., Van Den Akker, E., Forlani, S., De Graaff, W., Oosterveen, T., Roelen, B., et al. (1999). Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. International Journal of Developmental Biology, 43, 635–650.
Deschamps, J., & Van Nes, J. (2005). Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development, 132(13), 2931–2942.
Gaunt, S. J. (1994). Conservation in the Hox code during morphological evolution. International Journal of Developmental Biology, 38(3), 549–552.
Gaunt, S. J. (2000). Evolutionary shifts of vertebrate structures and Hox expression up and down the axial series of segments: a consideration of possible mechanisms. International Journal of Developmental Biology, 44, 109–117.
Guinard, G., Marchand, D., Courant, F., Gauthier-Clerc, M., & Le Bohec, C. (2010). Morphology, ontogenesis and mechanics of cervical vertebrae in four species of Penguins (Aves: Spheniscidae). Polar Biology, 33, 807–822.
Horan, G. S., Ramirez-Solis, R., Featherstone, M. S., Wolgemuth, D. J., Bradley, A., & Behringer, R. R. (1995). Compound mutants for the paralogous Hoxa-4, Hoxb-4, and Hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes and Development, 9, 1667–1677.
Horan, G. S. B., Wu, K., Woldgemuth, D. J., & Behringer, R. R. (1994). Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proceedings of the National Academy of Science USA, 91, 12644–12648.
Huang, R., Zhi, Q., Patel, K., Wilting, J., & Christ, B. (2000). Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anatomy and Embryology, 202(5), 375–383.
Ikeya, M., & Takada, S. (2001). Wnt-3a is required for somite specification along the anteropsoterior axis of the mouse embryo and for regulation of Cdx-1 expression. Mechanical Development, 103, 27–33.
Jadwisczak, P. (2006). Eocene penguins of Seymour Islands, Antarctica: Taxonomy. Polish Polar Research, 21(1), 3–62.
Kant, R., & Goldstein, R. S. (1999). Plasticity of axial identity among somites: Cranial somites can generate vertebrae without expressing Hox Genes appropriate to the trunk. Developmental Biology, 216, 507–520.
Kessel, M., Bailling, R., & Gruss, P. (1990). Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell, 61(2), 301–308.
Kessel, M., & Gruss, P. (1991). Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell, 67, 89–104.
Ksepka, D. T., Clarke, J. A., DeVries, T. J., & Urbina, M. (2008). Osteology of Icadyptes salasi a giant penguin from the Eocene of peru. Journal of Anatomy, 213, 131–147.
Marshall, H., Morrison, M. H., Studer, M., Pöpperl, H., & Krumlauf, R. (1996). Retinoids and Hox genes. The Federation of American Societies for Experimental Biology Journal, 10, 969–978.
Morgan, B. A. (1997). Hox genes and embryonic development. Poultry Science, 76, 96–104.
Nowicki, J. L., & Burke, A. C. (2000). Hox genes and morphological identity: Axial versus lateral patterning in the vertebrate mesoderm. Development, 127, 4265–4275.
Oostra, R. J., Hennekam, R. C. M., De Rooij, L., & Moorman, A. F. M. (2005). Malformations of the axial skeleton in museum Vrolik I: homeotic transformations and numerical anomalies. American Journal of Medical Genetics, 134A, 268–281.
Simpson, G. G. (1946). Fossil penguins. Bulletin of the American Museum of Natural History, 87.
Slack, K. E., Jones, C. M., Ando, T., Harrison, G. L. (Abby), Fordyce, R. E., Arnason, U., & Penny, D. (2006). Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Molecular Biology and Evolution, 23(6), 1144–1155.
Suemori, H., & Noguchi, S. (2000). Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Developmental Biology, 220, 333–342.
Wellik, D. M. (2007). Hox patterning of the vertebrate axial skeleton. Developmental Dynamics, 236, 2454–2463.
Young, T., Rowland, J. E., Van de Ven, C., Bialecka, M., Novoa, A., Carapuco, M., et al. (2009). Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Developmental Cell, 17, 516–526.
Acknowledgments
Carolina Acosta Hospitaleche and Claudia Tambussi (Museo de La Plata), as well as Eduardo Ruigómez (Museo Egidio Feruglio Paleontólogico), for providing photographs of the cervical vertebrae of Madrynornis mirandus, thus allowing the study of this fossil species. Daniel Ksepka (North Carolina State University) for the transmission of additional photographs of the cervical V of Icadyptes salasi, in order to confirm the first impressions on this important vertebra. Rémi Laffont (Université de Bourgogne) for his help in providing some references, René Guinard for checking the manuscript and the department of comparative anatomy of the Muséum National d’Histoire Naturelle de Paris for access to the osteological collections. Benedikt Hallgrimsson—the editor in chief—and two anonymous reviewers, whose very pertinent comments have helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guinard, G., Marchand, D. Modularity and Complete Natural Homeoses in Cervical Vertebrae of Extant and Extinct Penguins (Aves: Sphenisciformes). Evol Biol 37, 210–226 (2010). https://doi.org/10.1007/s11692-010-9097-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-010-9097-0